Graves’ Ophthalmopathy Treatment Market: A Billion-Dollar Opportunity For Pharma Companies
May 19, 2023
Table of Contents
Graves’ ophthalmopathy, also known as thyroid-associated ophthalmopathy and thyroid eye disease, is the most frequent extrathyroidal manifestation of Graves’ disease. The majority of Graves’ ophthalmopathy cases are caused due to Graves’ disease, and only a small percentage of these patients arise from other euthyroid or hypothyroid disorders.
As per DelveInsight’s estimates, as of 2022, ~2.4 million people were living with Graves’s ophthalmopathy across 7MM. The US contributed to the largest prevalent population of Graves’ ophthalmopathy, acquiring ~42% of the 7MM in 2022. Females constitute ~80% of the prevalent population. It tends to be more severe in men, in whom it occurs at an older age than in women. Moreover, 3% – 5% may develop sight-threatening diseases, such as compressive optic neuropathy or exposure keratopathy.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Thyroid awareness month
- Graves’ Disease Treatment Revolution: What’s Next in Line?
- FDA Approves Xolair for Food Allergies; FDA Accelerated Approval for Iovance’s AMTAGVI; Astellas ...
- Biologics and The Battle For Sight: How A New Wave of Therapies is Transforming Graves’ Orbitopat...
- Late-Stage Thyroid Eye Disease Treatments: 4 Prominent Therapies to Consider
The diagnosis and treatment rate of Graves’ ophthalmopathy is expected to increase in the coming years due to the increase in expected approvals of several pipeline candidates for Graves’ ophthalmopathy treatment, thus leading to an increase in awareness among patients and clinicians.

Current Pharmacological Options for Graves’ Ophthalmopathy Treatment
Current pharmacological management options for graves’ ophthalmopathy treatment target only 1/10th of the diagnosed population. Graves’ ophthalmopathy is managed using a combination of basic, medicinal, and surgical treatments, depending on the severity of the patient’s symptoms. The optimal Graves’ ophthalmopathy treatment goal is to reduce the risk of visual difficulties, minimize adverse effects, eliminate the need for surgical treatments, restore thyroid function, and prevent Graves’ ophthalmopathy from progressing or recurring.
Graves’ ophthalmopathy is often a single, self-limiting episode that can be separated into two phases based on the disease’s inflammatory activity. The acute, inflammatory phase normally responds to immunosuppressive anti-inflammatory medication, depending on the degree of involvement. Artificial tear drops can aid with dry eye relief in people with mild Graves’ ophthalmopathy. Supplementing with selenium can also be useful. If Graves’ ophthalmopathy is acute, a small number of individuals with mild Graves’ ophthalmopathy may be recommended for low-dose immunomodulation. It is critical to create an early immunosuppressive treatment that restricts the progression of the inflammatory illness in moderate-severe types. Thyroid disease management can enhance orbital involvement, while the course is largely independent in most individuals.
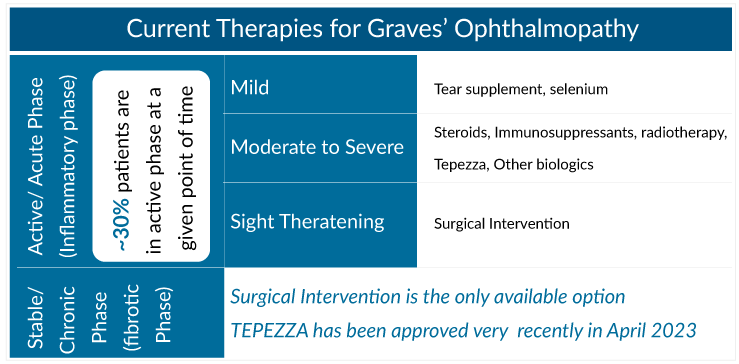
The pharmaceutical medications used to manage Graves’ ophthalmopathy are glucocorticoids, mycophenolate, rituximab, tocilizumab, teprotumumab, cyclosporine/mTOR inhibitors, and various additional forms of Graves’ ophthalmopathy treatment. Currently, teprotumumab is the only FDA-approved therapy for Graves’ ophthalmopathy treatment; for this reason, research into new drugs is unsurprisingly demanding.
TEPEZZA: One of the Most Successful Rare Disease Medicine Launches in History
TEPEZZA (teprotumumab) developed by Horizon Therapeutics is the only FDA-approved Graves’ ophthalmopathy treatment. TEPEZZA became the blockbuster therapy within two years of launch. Following a 24-week course of therapy, significant improvements in proptosis, diplopia, and quality of life have been observed. Teprotumumab is a completely human IgG1 monoclonal antibody directed against the human insulin-like growth factor-1 receptor; its metabolism has not been thoroughly characterized. However, it is expected to be degraded via proteolysis. It is a sterile, preservative-free, white-to-off-white lyophilized powder for IV infusions. It improved ptosis, diplopia, CAS score, and quality of life in patients with moderate-to-severe Graves’ ophthalmopathy and had a favorable safety profile.
Tepezza has become a go-to treatment for moderate to severe thyroid eye disease, with unseating orbital decompression as the most used Graves’ ophthalmopathy treatment. TEPEZZA witnessed a YoY growth of 103% within the 1st year of launch. As per DelveInsight Estimates, the drug will continue to grow at a CAGR of ~10% owing to its launch in EX-US geographies and label expansion to include chronic Graves’ ophthalmopathy patients. It is currently being studied in Japan as part of a Phase III (jRCT2031210453) trial to treat active Graves’ ophthalmopathy in Japanese patients.
Key Competitors in Pipeline for Graves’ Ophthalmopathy Treatment
Graves’ ophthalmopathy arena is finally beginning to evolve. New Graves’ ophthalmopathy drugs with the potential to provide sustained benefits are expected to contribute to the exponential Graves’ ophthalmopathy treatment market growth. The drug candidates by key players, such as Immunovant Sciences (batoclimab), Novartis Pharmaceuticals (secukinumab), Viridian Therapeutics (VRDN-001), Sling Therapeutics (linsitinib), Regeneron Pharmaceuticals (aflibercept), ValenzaBio (VB421), and others under late- and mid-phase clinical development have the potential to create a significant positive shift in Graves’ ophthalmopathy market size. The launch of emerging therapies for Graves’ ophthalmopathy treatment is expected during the forecast period of 2023–2032.
Batoclimab (Immunovant Sciences) is being studied for the treatment of severe autoimmune diseases caused by pathogenic immunoglobulin G (IgG), such as Graves’ ophthalmopathy. It was evaluated in an open-label, single-arm Phase IIa clinical trial for Graves’ ophthalmopathy treatment in 2019. The majority of participants (four of seven) tested at the end of treatment improved their clinical activity score (CAS) by two points. Three of the seven participants were IMVT-1401 proptosis responders. Later, the company achieved FDA alignment, and a Phase IIb trial was initiated and halted in February 2021 due to elevated total cholesterol and low-density lipoprotein levels observed in some trial subjects treated DA Division of Ophthalmology to move forward in Graves’ ophthalmopathy and initiated two Phase III (NCT05524571, NCT05517421) clinical trials to evaluate batoclimab to treat Graves’ ophthalmopathy Top-line findings from both Phase III trials are expected in the first half of 2025.
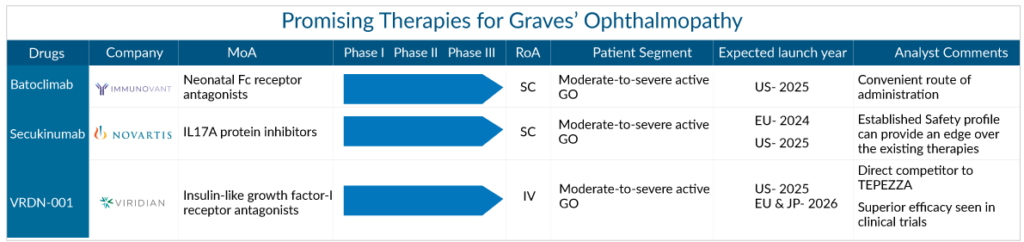
COSENTYX (secukinumab) developed by Novartis is an immunosuppressant agent. It is manufactured as a freeze-dried powder solution and solution in a prefilled syringe or pen for SC delivery. It is a completely human monoclonal anti-IL-17A recombinant antibody that is now licensed for the treatment of three inflammatory/autoimmune diseases: moderate-to-severe plaque psoriasis (PsO), psoriatic arthritis (PsA), and axial spondyloarthritis (axSpA). The drug is currently being studied in a Phase III (NCT04737330) trial to determine the efficacy and safety of secukinumab in adult patients with active, moderate-to-severe thyroid eye disease.
VRDN-001, Viridian’s lead product candidate, is a differentiated monoclonal antibody that targets the insulin-like growth factor-1 receptor (IGF-1R), a clinically and commercially recognized target for the treatment of Graves’ disease. The medicine is currently being tested in a Phase II/III (NCT05176639) trial to determine its safety, tolerability, and efficacy in active Graves’ ophthalmopathy patients.
Future Graves’ Ophthalmopathy Treatment Looks Promising
Graves’ ophthalmopathy treatment market is expected to see multiple approvals in the near future. DelveInsight estimates that the Graves’ ophthalmopathy market is expected to grow from approximately USD 2 billion in 2022 at a significant ~15% CAGR by 2032. As per the estimates ~98% of this revenue is attributed to the sales of TEPEZZA in the US.
In addition, recent attempts to raise patient and clinician awareness of Graves’ ophthalmopathy through various awareness programs are expected to result in earlier diagnosis and Graves’ ophthalmopathy treatment. Furthermore, the first step in ocular drug discovery programs is to design an effective molecule with an intended delivery strategy matched to the target compartment.
Moreover, the lack of disease-modifying therapeutic options for chronic Graves’ ophthalmopathy patients substantially influences their mental and social well-being. As a result, the dynamics of the Graves’ ophthalmopathy treatment market are anticipated to change in the coming years owing to the improvement in the diagnosis methodologies, raising awareness of the diseases, and incremental healthcare spending worldwide.

FAQs
Graves’ ophthalmopathy, also known as thyroid-associated ophthalmopathy and thyroid eye disease, is the most common extrathyroidal manifestation of Graves’ disease. It is an autoimmune disease in which the generation of autoantibodies causes inflammatory changes in the orbit. The autoantibodies are thought to be directed at a protein that is found in both the orbit and the thyroid.
Graves’ ophthalmopathy symptoms include eye irritation or grittiness, redness or inflammation of the conjunctiva, excessive tears or dry eyes, swelling of the eyelids, sensitivity to light, forward displacement or bulging of the eyes (called proptosis), and double vision.
The doctor may suggest scans, such as a computerized tomography (CT) scan or an MRI, to discover changes in the muscles around the eyes for Graves’ ophthalmopathy diagnosis. They may also need blood testing to see whether their thyroid hormone levels and antibodies are too high or too low.
The Graves’ ophthalmopathy treatment is determined by the patient’s symptoms and severity and includes basic, medicinal, and surgical treatments. The optimal Graves’ ophthalmopathy treatment goal is to reduce the risk of visual difficulties, minimize adverse effects, eliminate the need for surgical treatments, restore thyroid function, and prevent Graves’ ophthalmopathy from progressing or recurring.
Downloads
Article in PDF
Recent Articles
- Biologics and The Battle For Sight: How A New Wave of Therapies is Transforming Graves’ Orbitopat...
- Graves’ Disease Drug Pipeline: 6 Late-Stage Therapies to Watch
- FDA Approves Xolair for Food Allergies; FDA Accelerated Approval for Iovance’s AMTAGVI; Astellas ...
- Tepezza receives approval; a new way to treat Alzheimer’s
- Thyroid awareness month



