Understanding High Risk Smoldering Multiple Myeloma: A Bridge Between MGUS and Multiple Myeloma
Jul 23, 2024
Table of Contents
Smoldering Multiple Myeloma (SMM) is an asymptomatic condition that acts as an intermediate stage between monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma. First described by Kyle and Greipp in 1980, SMM typically affects individuals aged 50 to 70 and is usually diagnosed incidentally when an M protein is discovered during laboratory tests for various disorders.
What is High-Risk Smoldering Multiple Myeloma?
High-risk smoldering multiple myeloma (HRSMM) refers to SMM patients with a significantly higher likelihood of progressing to active multiple myeloma within two years. The progression risk ranges from 50-79% in the first two years. Biomarkers indicating higher risk include:
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Kiadis inks license deal with Sanofi; Gilead Ends Hep B Collaboration; Merck & Foghorn inks ...
- Abeona Secures FDA Nod for ZEVASKYN, the First Gene Therapy for RDEB; AbbVie Gains FDA Approval f...
- Regeneron Presents Positive Pivotal Data on Linvoseltamab for Relapsed/Refractory Multiple Myelom...
- 5 Most Promising CAR T-cell Therapies for Multiple Myeloma in Development
- Analyzing the Most Promising Drugs That Will Lose Patent in the US & EU in 2022
- Clonal plasma cell bone marrow infiltration ≥10%
- Serum M-protein ≥3 g/dL
- Serum-free light chain ratio >0.125 or <8
Smoldering Multiple Myeloma Symptoms
While smoldering multiple myeloma (SMM) typically presents without symptoms, it can occasionally manifest as bone pain, especially in the back or ribs, due to bone lesions. Other signs may include unexplained fatigue, recurrent infections, or kidney problems, often detected through abnormal kidney function tests. Additionally, some individuals might experience numbness or weakness in the legs from spinal cord compression caused by bone fractures or lesions. Early diagnosis and monitoring are crucial to manage these symptoms and potentially delay progression to active multiple myeloma.
Growing High-Risk Smoldering Multiple Myeloma Prevalence and Challenges
The global population of HRSMM is expected to grow remarkably, with the US projected to have the highest number of cases. Despite this increase, there is currently no approved drug for the treatment of high-risk smoldering multiple myeloma, highlighting a significant unmet medical need. Challenges in diagnosis, such as misdiagnosis and delayed detection, further complicate treatment efforts. Improving diagnostic techniques could prove vital in addressing these issues.
Treatments for Multiple Myeloma
The multiple myeloma treatment has evolved significantly, offering a range of options to manage and treat the disease effectively. Chemotherapy remains a cornerstone, utilizing powerful drugs to target rapidly dividing cancer cells, often in combination with corticosteroids like dexamethasone and prednisone to enhance treatment effects.
Advanced therapies include CAR T-cell therapy, such as idecabtagene vicleucel (ABECMA) and bispecific T-cell engagers (BiTEs), which redirect T cells to target and destroy myeloma cells. These therapies represent substantial advancements in cancer treatment, providing more tailored and effective options for patients.
Key Drugs for Multiple Myeloma Treatment
Key drugs like Bortezomib (VELCADE) and Carfilzomib (KYPROLIS), both proteasome inhibitors, are fundamental in multiple myeloma treatment regimens. These drugs disrupt proteasome function within cancer cells, leading to cell death and are often combined with other therapies for enhanced efficacy.
Chemotherapy continues to play a crucial role, with drugs like Carmustine (BiCNU) used in high-dose regimens before stem cell transplants. Targeted therapies, such as Daratumumab (DARZALEX) and Isatuximab (SARCLISA), which target CD38, and Ciltacabtagene Autoleucel (CARVYKTI), a CAR T-cell therapy targeting BCMA, are also pivotal in treating relapsed or refractory cases.
Monoclonal antibodies like Elotuzumab (EMPLICITI), which targets SLAMF7, and Immunomodulatory Drugs (IMiDs) such as Lenalidomide (REVLIMID) and Pomalidomide (POMALYST) have significantly improved outcomes by boosting the immune response against cancer cells. Thalidomide, the first IMiD used in myeloma treatment, although less common today, still plays a role in specific treatment strategies.
Treatments Focusing on High-Risk Smoldering Multiple Myeloma
While many drugs are approved or in the pipeline for multiple myeloma, some therapies are explicitly focused on the treatment of high-risk smoldering multiple myeloma.
Approved Drugs for High-Risk Smoldering Multiple Myeloma:
- DARZALEX (daratumumab): Johnson & Johnson
DARZALEX, a CD38-directed monoclonal antibody, was approved by the FDA in 2020 to treat high-risk smoldering multiple myeloma based on positive results from the CENTAURUS study.
The CENTAURUS study showed that DARZALEX significantly improved progression-free survival compared to observation in patients with high-risk smoldering multiple myeloma.
- EMPLICITI (elotuzumab): Bristol-Myers Squibb
EMPLICITI, an immunostimulatory antibody that targets SLAMF7, was approved by the FDA in 2015 for treating multiple myeloma, including in the high-risk smoldering setting.
The approval was based on the ELOQUENT-1 study, which demonstrated improved progression-free survival with Empliciti plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with high-risk smoldering multiple myeloma.
- NINLARO (ixazomib): Takeda
NINLARO, an oral proteasome inhibitor, was approved by the FDA in 2015 for the treatment of multiple myeloma, including in the high-risk smoldering setting.
The approval was supported by the TOURMAINE study, which showed that the addition of NINLARO to lenalidomide and dexamethasone improved progression-free survival in patients with high-risk smoldering multiple myeloma.
These targeted therapies are crucial in delaying disease progression and improving outcomes for patients with high-risk smoldering multiple myeloma, a stage that can progress to active multiple myeloma. Ongoing research continues to explore combination approaches and novel agents to enhance treatment options for this patient population further.
Pipeline Therapies for High-Risk Smoldering Multiple Myeloma:
Linvoseltamab (REGN5458): Regeneron
Linvoseltamab is a BCMAxCD3 bispecific antibody developed by Regeneron for patients with relapsed/refractory multiple myeloma (RRMM). It is designed to bind to BCMA on multiple myeloma cells and the CD3 receptor on T cells, thereby bridging them and activating T-cell-mediated killing of cancer cells.
The clinical development program for linvoseltamab includes several trials, notably a Phase III confirmatory trial (LINKER-MM3) currently enrolling patients with RRMM. Furthermore, Regeneron is exploring linvoseltamab in earlier lines of therapy and various disease stages. Notably, a Phase II trial is investigating its use in high-risk smoldering multiple myeloma, aiming to intervene at a critical juncture before full-blown multiple myeloma develops. This trial could significantly impact the treatment paradigm for high-risk smoldering multiple myeloma by potentially delaying or preventing progression to active disease.
Linvoseltamab leverages Regeneron’s ‘human antibody mouse’ technology (VelocImmune) and the ‘full-length bispecific antibody’ platform (VelociBi). The drug is currently undergoing several clinical trials:
- Phase III Confirmatory Trial (LINKER-MM3): Enrolling patients with R/R MM.
- Phase I/II Trial: Investigating use in the first-line setting.
- Phase II Trials: Focusing on high-risk smoldering multiple myeloma and monoclonal gammopathy of undetermined significance (MGUS).
- Combination Therapy: A planned Phase I trial with a Regeneron CD38xCD28 costimulatory bispecific in MM.
In February 2024, the FDA accepted the Biologics License Application (BLA) for linvoseltamab for priority review to treat adult patients with RRMM after at least three prior therapies. The target decision date is August 22, 2024.
Recent Research and Expert Insights
Studies indicate that patients with high-risk smoldering myeloma have a 75% chance of progressing to symptomatic multiple myeloma within five years, with a median time to progression of less than two years. At ASH 2023, Dr. Elizabeth Hill from the National Cancer Institute presented data from a Phase II trial where high-risk SMM patients received carfilzomib, lenalidomide, and dexamethasone for eight 28-day cycles, followed by two years of lenalidomide maintenance therapy (KRd-R). The trial showed promising results, with manageable low-grade toxicities and no grade 4 non-hematologic adverse events.
Dr. Manni Mohyuddin from the Huntsman Cancer Institute at the University of Utah commented on the controversy surrounding the treatment of high-risk multiple myeloma: “Given that there is no single or easy curative approach for myeloma yet, significant controversy exists about whether smoldering myeloma should be treated or not. Are we simply starting treatment early and giving more toxicity, or is there a real benefit to early treatment? These questions cause a lot of controversy, and many have different opinions.”
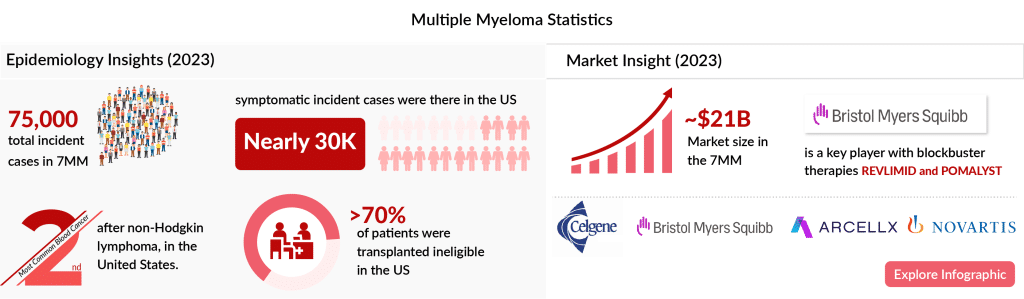
Multiple Myeloma Market Insights
The market for multiple myeloma treatments in the seven major markets was estimated at nearly USD 21.3 billion in 2023. This market is expected to grow positively by 2034 due to:
- Rising incident cases.
- Label expansion and penetration of current therapies.
- High adoption of newer therapies, especially CAR-T cell therapies and anti-BCMA.
- A rich emerging pipeline.
- Increased investment in R&D activities.
Here’s an infographic on multiple myeloma to gain deeper insights into the disease
Conclusion
High-risk smoldering multiple myeloma remains a significant area of unmet medical need. Ongoing research and clinical trials, such as those involving linvoseltamab, offer hope for better management and treatment options. Patients and healthcare providers can make more informed decisions and improve outcomes in managing this challenging condition by staying informed about the latest multiple myeloma research.

Downloads
Article in PDF
Recent Articles
- AbbVie Presents Phase III CANOVA Study Results; Novartis’ Iptacopan Shows Promise in Phase III St...
- Transforming Multiple Myeloma Treatment: The Promise of Novel Drug Classes
- DARZALEX FASPRO Regimen Approval: Johnson & Johnson Howling Success in Multiple Myeloma
- Pfizer/ OPKO Health’s Somatrogon; FDA EU for SCONE device; Antengene ATG-010 for rrMM and rrDLBCL...
- Revolutionary Advances and Bright New Horizons in Multiple Myeloma Treatment




