Entry of Izervay: First Geographic Atrophy Treatment to Slow Progression
Aug 18, 2023
Table of Contents
Globally, more than 5 million people are affected by geographic atrophy. Also, as per the published study by the GER group, the global prevalence of geographic atrophy is 0.66% in all ages, but it occurs in 0.34% for 65–74 years old, 1.3% for 75–84, and 4.4% over 85 years old. Geographic atrophy is a progressive, irreversible, and blinding disease that tends to affect both eyes. Nearly two-thirds of geographic atrophy cases are reported in people aged 80 years and above. GA is responsible for ~20% of all cases of legal blindness in the US and 26% of cases in the UK. According to the “American Macular Degeneration Foundation,” approximately 85–90% of the cases of macular degeneration are the “dry” (atrophic) type.
As per the assessment done by DelveInsight, in the 7MM, the total diagnosed cases of geographic atrophy were ~2.8 million cases in 2021. These cases are expected to grow with a significant CAGR in the study period (2019–2032). As per the estimates, in age-specific cases of geographic atrophy, the most number of cases (i.e., nearly two-thirds) was seen in patients of age 70–79 years, followed by the age group of ≥ 80 years and 60–69 years.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- RegeneRx’s Phase 3 Neurotrophic Keratitis Clinical Trial of RGN-259; Aldeyra’s Phase 3 INVIGORATE...
- Dry AMD Treatment- An Underserved Market With Billion-Dollar Potential
- Mapping The Pipeline Development In The Geographic Atrophy Market
- Edwards’ Sapien 3 with Alterra Prestent; Koios Medical’s breast, thyroid cancer-spotting AI; Line...
- EU Approves Galderma’s NEMLUVIO for Atopic Dermatitis and Prurigo Nodularis; GSK’s Penmenvy Wins ...
Currently, there are not so many approved treatments present for geographic atrophy treatment. The Age-Related Eye Disease Study (AREDS) study suggested that supplementing the diet of patients with vitamin C, vitamin E, beta-carotene and zinc could significantly reduce the risk of progressing to advanced AMD and sometimes low visual aids and counseling are also helpful. Several geographic atrophy therapies which reached an advanced stage of development are already failed, however, currently, several therapies are in the pipeline to prevent the progression of geographic atrophy.

Entry of Izervay in the Geographic Atrophy Treatment Space
Iveric Bio’s intravitreal avacincaptad pegol, now marketed as Izervay, was approved by the FDA on 4 August, for the treatment of geographic atrophy secondary to age-related macular degeneration. The regulatory victory comes three months after Astellas Pharma paid $5.9 billion for the New Jersey biotech. The transaction was completed last month. According to Astellas, the approval makes Izervay the first authorized geographic atrophy medication that has significantly decreased GA progression at 12 months across two Phase III studies. The eye injection also provides a new therapy option for clinicians and patients across the United States, according to Iveric Bio President Pravin Dugel in a statement.Izervay is an intravitreally given inhibitor of the complement C5 protein that was discovered and developed by Iveric. By preventing C5 cleavage, the eye injection disturbs other downstream processes, such as the creation of the membrane attack complex and inflammasome, which causes the typical symptoms of GA.
We are overjoyed to have received FDA approval for IZERVAY and to be able to offer a new therapy to physicians and appropriate patients in the United States. In this devastating progressive disease, time, vision, and safety all matter.” We’d want to thank everyone who helped us attain this milestone and keep our promise to pioneer revolutionary therapeutics for retinal disorders.
Pravin U. Dugel, MD, President, Iveric Bio, An Astellas Company
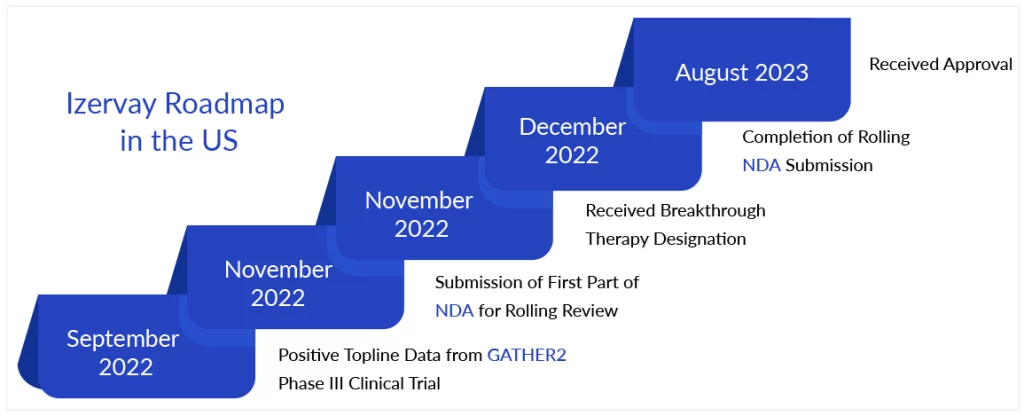
Iveric investigated the therapeutic potential of Izervay’s MoA in the Phase III GATHER1 and GATHER2 studies. GATHER1, a prospective, randomized, and sham-controlled pivotal Phase II/III trial, found that at the 2-mg dose level, Izervay decreased GA growth by 27.4% over 12 months, while the 4-mg dose was somewhat more effective at 27.8%. Both dosage levels were statistically superior to sham. In September 2022, the company announced that topline data from the Phase III GATHER2 study had satisfied the study’s prespecified primary aim. At 12 months, individuals treated with Izervay had considerably slower growth in the GA area when compared to sham controls.
Izervay demonstrated an acceptable safety profile in both investigations. The most prevalent side effect reported in individuals treated with Izervay across the GATHER program was bleeding beneath the clear lining of the eye, followed by increased intraocular pressure and impaired vision. Astellas will charge $2,100 for a monthly dose of Izervay and expects it to be available in the United States in two to four weeks. The corporation is currently “reviewing potential financial impacts of this approval” for its current fiscal year, which ends in March of next year.
Will Apellis’ Syfovre Safety Concerns Give Iveric’s Izervay an Edge?
According to the American Society of Retina Specialists’ Research and Safety in Therapeutics Committee, Apellis’ recently approved eye injection Syfovre (pegcetacoplan injection) has been linked to several cases of retinal vasculitis, a rare complication involving eye inflammation, as reported by several media outlets. Apellis has conducted a thorough examination of these issues, including a review of Phase III safety data from Syfovre’s research program as well as its manufacturing process. However, “there is no indication that the drug product or manufacturing issues contributed to these events,” according to the company’s announcement.
Syfovre was approved as the first treatment for geographic atrophy secondary to age-related macular degeneration in February 2023. The complement inhibitor medication works by targeting C3, the primary participant in the complement cascade, and thereby controlling downstream immune responses. Apellis has not officially verified these incidents of blindness, but the company did publish efficacy data for the eye injection at the ASRS annual meeting as well. Syfovre continued to minimize lesion growth in the GALE extension study after 30 months of continuous treatment. Apellis also released its second-quarter results report recently, emphasizing Syfovre’s $67.3 million in sales, which account for more than half of the company’s net product revenue for the quarter.
Promising Players in Line for Geographic Atrophy Treatment
The dynamics of the geographic atrophy treatment market are anticipated to change in the coming years owing to the improvement in the diagnosis methodologies, raising awareness of the disease, incremental healthcare spending across the world, and the expected launch of emerging therapies during the forecast period. Key players like Genentech/Roche (RO7171009; RG6147), Ionis Pharmaceuticals/Roche (IONIS-FBLRx), NGM Biopharmaceuticals (NGM621), Aviceda Therapeutics (AVD-104), Stealth BioTherapeutics (Elamipretide), CellCure Neurosciences/Lineage Cell Therapeutics and Roche (OpRegen), Novartis (PPY988/GT005), Regenerative Patch Technologies (CPCB-RPE1), and others are involved in developing novel geographic atrophy treatments.
Aviceda Therapeutics developing AVD-104 an intravitreal nanoparticle molecule with a unique dual mechanism of action for geographic atrophy treatment through its modulation of critical inflammatory pathways via inhibition of the activity of retinal macrophages and repolarization of activated macrophages to their resolution state coupled with inhibition of complement cascade amplification. Recently in April 2023, AVD-104 received FDA clearance for the Investigational New Drug (IND) application for initiating Phase II (SIGLEC trial) clinical trial and is expected to begin in Q2 2023.
NGM621 by NGM Biopharmaceuticals is a humanized Immunoglobulin 1, or IgG1, monoclonal antibody administered via intravitreal, or IVT, injection. NGM621 was engineered to potently bind to and be a long-acting inhibitor of complement C3 with the treatment goal of reducing disease progression in patients with geographic atrophy, secondary to age-related macular degeneration. NGM Biopharmaceuticals announced it had completed enrollment in the Phase II CATALINA study, which is evaluating the safety and efficacy of intravitreal injections of NGM621 in patients with geographic atrophy secondary to age-related macular degeneration. Data from a Phase I trial showed that NGM621 was well tolerated, with no patients experiencing serious SAEs or drug-related adverse events. In February 2022, NGM621 received Fast Track designation from the US FDA, for secondary to age-related macular degeneration. If approved, the medicine will have a chance to differentiate itself in geographic atrophy treatment and opportunities to establish category leadership and the potential for every 8-week dose. In October 2022, NGM announced Phase II data. According to the data trial did not meet the primary endpoint of a statistically significant rate of change in the GA lesion area using slope analysis over 52 weeks for NGM621 versus sham.
Novartis, PPY988 previously known as GT005 involves a new technique of putting copies of a gene producing a proprietary protein developed by Gyroscope, which counteracts the inflammation caused by the complement system, into the cells of the retina to help them function normally. In February 2022, Gyroscope Therapeutics was acquired by Novartis. The drug has received Fast Track Designation from the FDA for geographic atrophy treatment secondary to dry AMD. According to interim results from the Phase I/II (FOCUS) trial, there were no dose-related trends in the frequency or type of adverse events and no GT005-related serious adverse events. The company expects to file regulatory submissions in GA in 2026.
Belite Bio, a subsidiary of Lin BioScience is developing LBS-008 for the treatment of dry AMD. Administration of LBS-008 leads to reduced levels of retinol-binding protein (RBP) which, in turn, leads to decreased levels of vitamin A in the eye and reduced accumulation of the toxic A2E and thus successfully prevents the formation of the toxins associated with macular degeneration. In June 2020, Belite Bio Announced Positive results from the phase I clinical trials of LBS-008. LBS-008 has been granted Fast Track Designation, rare pediatric disease designation (RPD) in the US, and orphan drug designation (ODD) in the US and Europe.
To summarize, given the large number of potential medicines being studied for geographic atrophy treatment, it is reasonable to expect that the geographic atrophy treatment space will undergo significant change during the forecast period of 2023–2032.
FAQs
Geographic atrophy is a severe form of age-related macular degeneration (AMD). Macular degeneration, commonly known as age-related macular degeneration, is a medical disorder that causes blurred or no vision in the center of the visual field. There are two types of macular degeneration: “dry” and “wet.”
Symptoms of geographic atrophy can vary from mild to severe and usually develop slowly over time. Common signs include blurred or distorted central vision, dark or blank spots in the visual field, and difficulties with tasks that require detailed vision. Peripheral vision is generally unaffected, allowing individuals to maintain some level of functional vision.
Diagnosing geographic atrophy involves comprehensive eye examinations and imaging tests. Ophthalmologists often use optical coherence tomography (OCT) to visualize the layers of the retina and assess the extent of retinal damage. Fluorescein angiography may also be employed to detect abnormal blood vessel growth and leakage.
The geographic atrophy treatment options include nutritional supplements, such as high-dose antioxidants and zinc, which have shown potential in some cases. Additionally, ongoing research is exploring potential therapies, such as stem cell transplantation and gene therapy, to target the underlying causes of geographic atrophy.

Downloads
Article in PDF
Recent Articles
- Geographic Atrophy Treatment Market: Novel Therapies to Enter the Market
- EU Approves Galderma’s NEMLUVIO for Atopic Dermatitis and Prurigo Nodularis; GSK’s Penmenvy Wins ...
- RegeneRx’s Phase 3 Neurotrophic Keratitis Clinical Trial of RGN-259; Aldeyra’s Phase 3 INVIGORATE...
- Mapping The Pipeline Development In The Geographic Atrophy Market
- Actinium Announces Phase III SIERRA Trial Results; FDA Approves Apellis’s Geographic Atrophy Drug...



