Leptomeningeal Metastases Treatment Challenge: High Burden, Zero Approvals
Dec 29, 2025
Table of Contents
Leptomeningeal metastases, also known as leptomeningeal disease, carcinomatous meningitis, or neoplastic meningitis, represent a serious complication of advanced cancer in which malignant cells spread through the cerebrospinal fluid (CSF) to infiltrate the protective membranes surrounding the brain and spinal cord. While this condition affects approximately 5-8% of patients with metastatic solid tumors, undiagnosed cases may be considerably more common. Any cancer can potentially cause leptomeningeal metastases, but the condition is most frequently associated with lung cancer, breast cancer, melanoma, and gastrointestinal malignancies.
Understanding the Disease Burden
The burden of leptomeningeal metastases remains substantial across major healthcare markets. According to epidemiological data, approximately 110,000 new leptomeningeal metastases cases are diagnosed annually in the United States alone. Among cancer types, melanoma carries the highest frequency of leptomeningeal involvement, affecting 22-46% of patients, followed by small cell lung cancer, breast cancer, non-small cell lung cancer, and head and neck cancers. This high incidence makes effective treatment strategies critically important for improving patient outcomes and quality of life.
Downloads
Click Here To Get the Article in PDF
The clinical presentation of leptomeningeal metastases is highly variable, often involving multiple neurological manifestations termed “multifocal deficits.” Patients may experience encephalopathy, radiculopathy, cranial nerve dysfunction, and spinal cord symptoms. The diffuse nature of the disease frequently results in multiple noncontiguous lesions, making diagnosis particularly challenging. Liquid biopsy techniques, including detection of circulating tumor cells (CTCs) and cell-free tumor DNA (cfDNA) in CSF, are emerging as innovative diagnostic advancements that enhance accuracy and enable identification of targetable mutations.
The Therapeutic Challenge: Blood-Brain Barrier Penetration
One of the primary challenges in treating leptomeningeal metastases is the blood-brain barrier (BBB), which significantly limits the penetration of most systemic chemotherapy agents into the cerebrospinal fluid. This physiological barrier requires innovative therapeutic strategies that can either bypass it entirely or utilize agents with superior CNS bioactivity. Understanding the mechanisms and limitations of different treatment modalities is essential for selecting appropriate therapeutic approaches for individual patients.
Current Leptomeningeal Metastases Treatment Paradigm
Currently, there are no FDA-approved leptomeningeal metastases drugs specifically targeting the disease. The management of leptomeningeal metastases primarily involves a multimodal approach, including radiation therapy to shrink tumors and chemotherapy for more aggressive cases. Symptom management, such as pain relief, physical therapy, and nutritional support, is also crucial for improving patients’ quality of life.”
Patients with poor prognostic indicators, such as significantly reduced quality of life, irreversible neurological deficits, encephalopathy from widespread LM involvement, or uncontrolled systemic disease, are generally best managed with a palliative strategy, even when LM-directed therapies are considered. Regardless of the treatment pathway, supportive care remains essential for mitigating neurological symptoms in all individuals with LM.
According to the 2022 NCCN Guidelines (updated 2023), patients with favorable clinical features may receive stereotactic radiosurgery (SRS), involved-field radiotherapy (IFRT), or whole-brain radiotherapy (WBRT), in combination with systemic therapy and/or intrathecal (IT) chemotherapy. For those with unfavorable features, including bulky CNS disease, palliative IFRT is an option.
Surgical management primarily includes ventriculoperitoneal shunting (VPS) to treat symptomatic hydrocephalus and placement of a ventricular access device, such as an Ommaya or Rickham reservoir, for IT chemotherapy delivery. When both a VPS and an Ommaya reservoir are required, an on–off valve can help balance CSF pressure control with effective drug administration. However, VPS procedures carry risks such as tumor spread, device malfunction, and infection.
Craniospinal irradiation (CSI) provides broad radiotherapy coverage for LM but is used sparingly in adults due to prior radiation exposure and compromised bone marrow reserves from chemotherapy, which increase toxicity. Newer CSI techniques, including tomotherapy and proton therapy, may reduce toxicity but need further validation.
Intrathecal chemotherapy remains a cornerstone of LM treatment because the blood–brain barrier restricts the efficacy of many systemic agents. IT therapy can be delivered via lumbar puncture or through an Ommaya catheter for more consistent CSF access. Systemic chemotherapy also plays an important role in treating both LM and systemic disease, though agents must be lipid-soluble or given at high doses to reach therapeutic CSF concentrations. Drugs like methotrexate and cytarabine often require dose escalation due to limited CNS penetration.
Sparse Industry Engagement in Leptomeningeal Metastases Therapies
Pharmaceutical involvement in leptomeningeal metastases has historically been modest, with most companies limiting their role to providing investigational compounds or partial financial support for academic-led studies rather than driving comprehensive leptomeningeal metastases drug development efforts.
The developing treatment landscape for leptomeningeal metastases has encountered notable hurdles. Y-mAbs Therapeutics’ Omburtamab, a radiolabeled monoclonal antibody directed against B7-H3 for CNS tumors and LM, completed its pivotal trials but was issued a Complete Response Letter (CRL) by the FDA in 2022. The agency cited insufficient evidence of clinical benefit and concerns regarding the study’s design.
This situation is unprecedented, driven not by regulatory convention but by newly emerging clinical insights. It signals a major turning point in which therapeutic progress for leptomeningeal metastases is being guided by compelling efficacy data rather than by historical treatment norms.
Still, a few notable exceptions stand out. Plus Therapeutics is advancing REYOBIQ, a targeted radiotherapeutic specifically engineered for LM, while Angiochem previously progressed ANG1005, a peptide-drug conjugate capable of crossing both the blood–brain and blood–CSF barriers, though its development has since paused. Whereas, Kazia Therapeutics and Genentech are working on their drug candidate, Paxalisib.
REYOBIQ (rhenium Re186 obisbemeda) is a novel injectable radiotherapy intended to deliver concentrated, localized radiation to tumors within the central nervous system. Its design leverages the distinctive characteristics of rhenium-186, including its short half-life, beta emissions for tumor ablation, and gamma emissions that enable real-time imaging. This combination positions REYOBIQ as a promising candidate for CNS-directed treatment. The therapy is currently being evaluated for LM in the ongoing ReSPECT-LM clinical program.
In July 2025, Plus Therapeutics announced plans to present results from the ReSPECT-LM trial and host an industry-supported educational symposium at the SNO/ASCO CNS Metastases Conference scheduled for August 14–16, 2025. Earlier, in March 2025, the company reported that the FDA had granted Orphan Drug Designation to REYOBIQ for the treatment of LM in patients with lung cancer.
Paxalisib (GDC-0084) is another investigational agent under development for various brain cancers. It is a brain-penetrant inhibitor of the PI3K/Akt/mTOR pathway, a key regulator of cellular growth and proliferation. Unlike many drugs in its class, paxalisib is specifically formulated to cross the blood–brain barrier, enabling effective delivery into brain tissue—a critical feature for treating CNS malignancies.
At the 66th ASTRO Annual Meeting in October 2024, Kazia Therapeutics presented Phase I results showing that a 45 mg dose of paxalisib combined with radiotherapy achieved a 67% partial response rate in patients with brain or leptomeningeal metastases harboring PI3K mutations. Importantly, over two-thirds of patients treated at the maximum tolerated dose exhibited intracranial responses, surpassing historical efficacy rates for radiation therapy alone.
The anticipated launch of these emerging therapies are poised to transform the leptomeningeal metastases market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the leptomeningeal metastases market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
What Lies Ahead in Leptomeningeal Metastases Treatment?
Leptomeningeal metastases remain a challenging complication of advanced cancer with historically poor prognosis. However, the therapeutic landscape is evolving rapidly with advances in intrathecal drug delivery, CNS-penetrant systemic therapies, immunotherapy, and precision medicine approaches.
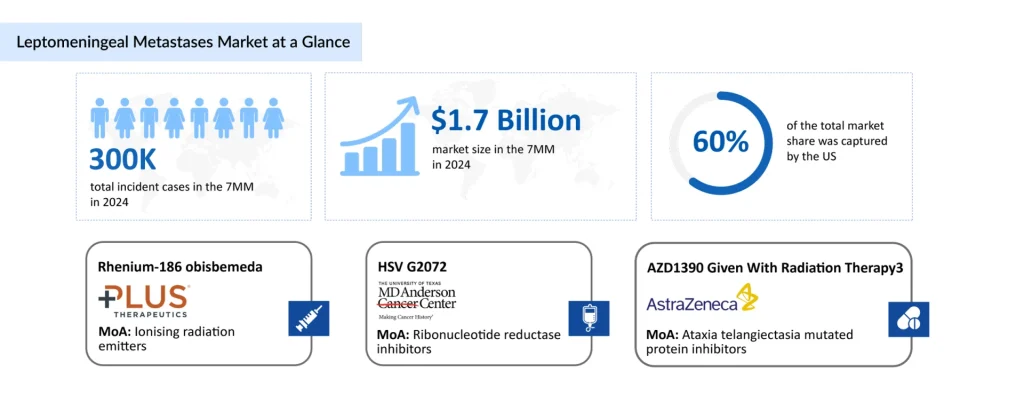
In 2024, the leptomeningeal metastases market size in 7MM was approximately USD 1.7 billion, which is expected to rise in 2034. In 2024, the US captured nearly 60% of the leptomeningeal metastases market, driven by extensive use of systemic, targeted, intrathecal, and radiotherapy-based interventions. This market dominance is supported by favorable reimbursement structures, rapid adoption of novel therapies, and a healthcare system that enables high-cost, aggressive treatment strategies.
Successful treatment of leptomeningeal metastases requires a multidisciplinary approach involving medical oncology, neuro-oncology, neurosurgery, and radiation oncology specialists, combined with comprehensive supportive care. Early identification and diagnosis, combined with prompt initiation of appropriate therapy, remain critical factors in optimizing outcomes. As research continues to advance our understanding of leptomeningeal disease pathophysiology and as new therapeutic agents continue to emerge, the prognosis for these patients will likely continue to improve.




