Top 8 Applications of Nanomedicine in Healthcare
Feb 04, 2026
Table of Contents
As we navigate, the medical landscape is undergoing a silent but profound transformation. We have officially moved beyond the era of “one-size-fits-all” medicine and entered the age of Precision Health, driven largely by the maturity of nanomedicine. While traditional medicine has long relied on macroscopic interventions, pills that circulate systemically or surgeries that remove entire sections of tissue, nanomedicine operates at the molecular scale.
By definition, nanomedicine involves the use of materials and devices engineered at the nanoscale (1-100 nm). To put this in perspective, a single sheet of paper is about 100,000 nanometers thick. At this microscopic threshold, the classical laws of physics often give way to quantum effects, and the surface-area-to-volume ratio of materials increases exponentially. This allows scientists to “program” particles to interact with biological systems in ways that were previously impossible.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Bristol Myers’ Opdivo combo Opdualag for Melanoma; Biogen’s Aduhelm; Marinus’ Ztalmy for CDKL-5 D...
- Fourth FDA Approval for AbbVie’s Vraylar; FDA Approves Ferring’s Adstiladrin for NMIBC; Merck and...
- Unleashing the Power of mRNA: A Revolutionary Approach to Vaccines and Therapeutics
- Nanomedicine’s Impact: Transforming the Future of Healthcare Industry Dynamics
- Nanomedicine Market: Evaluating the Pioneering Innovations and Future Growth Prospects
The journey has been marked by a shift from passive to active nanotechnologies. Early nanomedicine focused primarily on “passive targeting”, using the leaky blood vessels of tumors to let drug-carrying liposomes accumulate naturally. Today, we are witnessing the rise of smart, stimuli-responsive systems. These are nanomachines capable of sensing their environment, bypassing the body’s most guarded barriers (like the blood-brain barrier), and releasing therapeutic payloads only when they encounter specific biological “keys,” such as a certain enzyme or a specific pH level.
The Top 8 Applications of Nanomedicine in Modern Healthcare
Nanomedicine is already impacting nearly every branch of healthcare, demonstrating that it’s not merely a future concept but a technology actively shaping modern medical practice. In fact, a common question that arises is whether nanomedicine is currently in use, and the answer is yes. Its adaptability and strong performance underscore its fundamental role in contemporary medical innovation. Let’s dive into the key 8 applications of nanomedicine in healthcare.
Precision Oncology: The Era of Targeted Drug Delivery
Traditional chemotherapy is often described as a “sledgehammer” approach; it kills cancer cells, but it also devastates healthy tissue, leading to the debilitating side effects we’ve known for decades. Nanomedicine has introduced the “scalpel.”
In targeted oncology drug delivery, nanoparticles serve as sophisticated vehicles for transporting drugs. These carriers, often liposomes or polymeric nanoparticles, are engineered with ligands, molecules that act like keys to a lock. These “keys” only bind to specific receptors overexpressed on the surface of tumor cells.
The clinical anchor of this field is the use of stimuli-responsive nanocarriers. These particles don’t just find the tumor; they wait for a specific signal, such as the acidic pH of a tumor microenvironment or a specific internal temperature, to “burst” and release their toxic payload exactly where it’s needed. This minimizes systemic toxicity, allowing patients to undergo treatment with significantly fewer side effects like hair loss, nausea, or immune suppression.
The Genetic Frontier: LNPs, mRNA, and Beyond
The success of mRNA vaccines during the global pandemic was a “proof of concept” for Lipid Nanoparticles (LNPs). Today, this technology has expanded far beyond vaccines. LNPs are the primary delivery mechanism for gene therapies and mRNA therapeutics designed to treat rare genetic disorders and chronic diseases.
The challenge with genetic material (such as DNA or RNA) is that it is highly fragile and readily degraded by the body’s enzymes.LNPs act as a protective “lipid envelope,” shielding the genetic code until it reaches the target cell’s interior.
We are seeing the rise of multi-organ-targeting LNP platforms. While early versions primarily localized to the liver, newly engineered lipids enable researchers to target the lungs for cystic fibrosis treatment or the heart for regenerative post-infarction therapy. This is turning the body into its own “bioreactor,” capable of producing the very proteins it lacks due to genetic mutations.
Beyond Sight: Advanced Diagnostic Imaging
Early detection remains the holy grail of cancer treatment. Nanotechnology has advanced imaging beyond the capabilities of standard MRI or CT. By using Quantum Dots and Superparamagnetic Iron Oxide Nanoparticles (SPIONs), clinicians can now achieve “molecular imaging.”
These nanoparticles are designed to attach to the very first clusters of malignant cells, long before a visible tumor forms. When viewed under specialized imaging equipment, these particles glow or provide high-contrast signals, effectively “lighting up” cancer at the microscopic level.
Furthermore, AI-generated peptide sensors were introduced. These nanoprobes circulate in the bloodstream and bind to cancer-associated enzymes. When a reaction occurs, they release a synthetic “biomarker” that can be detected in a simple urine test, offering a non-invasive, high-sensitivity screening tool for at-home diagnostics.
Building Life: Regenerative Medicine and Tissue Engineering
When an organ fails or a bone is shattered, the traditional solution is a transplant or a metal implant. Nanomedicine offers a third way: Regeneration.
By creating nanofiber scaffolds that mimic the body’s natural extracellular matrix, scientists can provide a structure for stem cells to latch onto and grow. “Bio-active” scaffolds are the gold standard. These are not merely passive structures; they are embedded with growth factors released at a nano-programmed rate to instruct stem cells when to differentiate into muscle, bone, or nerve tissue.
We are also seeing the use of magnetic nanoparticles to physically guide stem cells to the site of an injury. For instance, in treating complex bone fractures, magnetic fields can “pull” healing cells into the fracture gap, accelerating recovery by up to 50% compared with traditional methods.
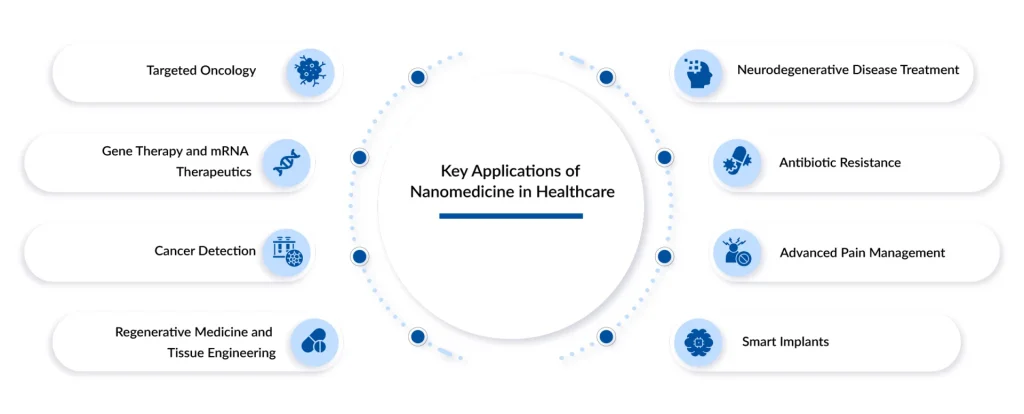
Crossing the Barrier: Treating Neurodegenerative Diseases
The Blood-Brain Barrier (BBB) is the body’s most formidable security system, designed to keep toxins out of the brain. Unfortunately, it also keeps out 98% of potential neurological drugs. This is why diseases like Alzheimer’s and Parkinson’s have been so difficult to treat.
Nanotechnology has developed “Trojan Horse” strategies to bypass this barrier. By coating nanoparticles with surfactants or specific proteins, they can trick the BBB’s transport systems into letting them pass.
Nasal-to-brain nano-delivery has become a breakthrough reality. Specialized nano-emulsions delivered via a nasal spray bypass the systemic circulation entirely, traveling along the olfactory nerves directly into the brain. This allows for high concentrations of neuroprotective drugs to reach the central nervous system without the need for invasive injections or high oral doses that could damage the liver.
The End of the Superbug: Nano-Antibiotics
As traditional antibiotics lose effectiveness against “superbugs,” nanomedicine has emerged with Nano-Antibiotics (nAbts). Unlike chemical drugs that target a single biological pathway (which bacteria can readily mutate to evade), nano-antibiotics often rely on physical forces.
Silver, gold, and zinc oxide nanoparticles possess intrinsic antimicrobial properties. They can physically puncture bacterial cell walls or generate “reactive oxygen species” (ROS) that shred the bacteria’s internal machinery.
Because these attacks are physical rather than purely biochemical, it is significantly harder for bacteria to develop resistance. These are used not only as injectable treatments for sepsis but also as nanocoatings on surgical tools and hospital surfaces to prevent the spread of infection at the source.
Precision Relief: Advanced Pain Management
Chronic pain is one of the leading causes of disability worldwide, yet our primary tools, opioids, carry a high risk of addiction and systemic side effects. Nanotechnology is revolutionizing Local Anesthesia and Pain Management by localizing the relief.
New nanocapacitor patches and injectable hydrogels can encapsulate pain-relieving drugs and release them slowly over weeks or even months. For a patient recovering from knee surgery, a single “nano-shot” into the joint can provide targeted numbing for the entire recovery period, eliminating the need for oral painkillers.
Furthermore, researchers are using nanoparticles to “silence” pain genes (using siRNA) at the site of chronic inflammation. This addresses the source of the pain signal rather than just masking it, providing a non-addictive path to long-term relief.
The Living Body: Real-Time Monitoring and Smart Implants
The final frontier of nanomedicine is the Smart Implant. Implants are no longer “dumb” pieces of metal or plastic; they are now integrated with nano-sensors and wireless connectivity.
A smart orthopedic implant can now monitor the mechanical load on a healing bone and transmit that data to a surgeon’s tablet in real-time. If the patient is over-exerting themselves, or if the bone isn’t fusing correctly, the doctor knows instantly.
Even more impressive are injectable nanobiosensors that circulate in the blood to monitor glucose, cholesterol, or cardiac troponin (an indicator of heart attack). These “nanoscale sentinels” provide a continuous stream of data, moving us from a “reactive” healthcare system (treating patients when they’re sick) to a “proactive” one (preventing illness before it starts).
The Road Ahead: Challenges and Opportunities
Despite its brilliance, the field faces significant hurdles. Regulatory harmonization remains a challenge; the FDA and EMA are still refining the “Drug-Device” classification for smart implants, which can delay clinical entry. Furthermore, the complexity of large-scale manufacturing, ensuring that every nanoparticle in a billion-dose batch has the exact same diameter, requires a level of quality control that traditional pharmaceutical plants are still rushing to adopt.
However, the trajectory is clear. We are moving toward a world in which “illness” is detected by a nanosensor before symptoms appear, and “treatment” is a targeted intervention that spares the rest of the body from harm. In the coming years, the revolution is no longer merely imminent; it is here and unfolding at the scale of the atom.
Conclusion: The Nano-Revolution is Here
The nano-revolution is here, quietly transforming the world at a scale too small to see but too powerful to ignore. Nanotechnology is reshaping medicine with targeted drug delivery, boosting sustainability through advanced materials, and driving innovation in electronics, energy, and environmental protection. From ultra-efficient batteries to self-healing materials, nanoscale science is unlocking possibilities once thought impossible.
The global nanomedicine market is projected to grow at a CAGR of 11.39% from 2025 to 2032. The demand for nanomedicine is primarily driven by the increasing prevalence of chronic diseases, including cardiovascular, neurological, inflammatory, and musculoskeletal diseases.
Additionally, the active participation of leading companies such as Sanofi, Pfizer Inc., Taiwan Liposome Company, Ltd., Johnson & Johnson, Bristol-Myers Squibb Company, Cytimmune Sciences, Inc., Luminex Corporation, Merck & Co., Inc., Starpharma Holdings Limited, Precision NanoSystems, Nanobiotix, Medtronic, Spago Nanomedical AB, Genetic Immunity, Nanospectra Biosciences, and others will further drive the growth of the nanomedicine market.
As this technology continues to evolve, it promises not just faster and smaller devices but also smarter, safer, and more sustainable solutions for global challenges, marking the beginning of a new era shaped by the tiniest building blocks of matter.

Downloads
Article in PDF
Recent Articles
- Nanomedicine Market: Evaluating the Pioneering Innovations and Future Growth Prospects
- Bristol Myers’ Opdivo combo Opdualag for Melanoma; Biogen’s Aduhelm; Marinus’ Ztalmy for CDKL-5 D...
- Nanomedicine’s Impact: Transforming the Future of Healthcare Industry Dynamics
- Fourth FDA Approval for AbbVie’s Vraylar; FDA Approves Ferring’s Adstiladrin for NMIBC; Merck and...
- Unleashing the Power of mRNA: A Revolutionary Approach to Vaccines and Therapeutics