Redefining PH-ILD Management: From Prostacyclins to Multi-mechanistic Innovations
Jul 11, 2025
Table of Contents
Pulmonary hypertension associated with interstitial lung disease (PH-ILD) is a serious, progressive complication characterized by increased Pulmonary Vascular Resistance (PVR) and disrupted gas exchange. This rise in PVR places extra stress on the right side of the heart, eventually leading to right heart dysfunction and failure if untreated. PH-ILD develops in patients with various fibrosing or inflammatory lung diseases that gradually scar lung tissue and damage the pulmonary vasculature.
The main underlying causes include Idiopathic Pulmonary Fibrosis (IPF), which is the most common and best-studied driver of PH-ILD, along with Nonspecific Interstitial Pneumonia (NSIP), sarcoidosis, connective tissue disease-associated ILDs, Combined Pulmonary Fibrosis and Emphysema (CPFE), hypersensitivity pneumonitis, and other less common fibrotic lung disorders.
Downloads
Click Here To Get the Article in PDF
Recent Articles
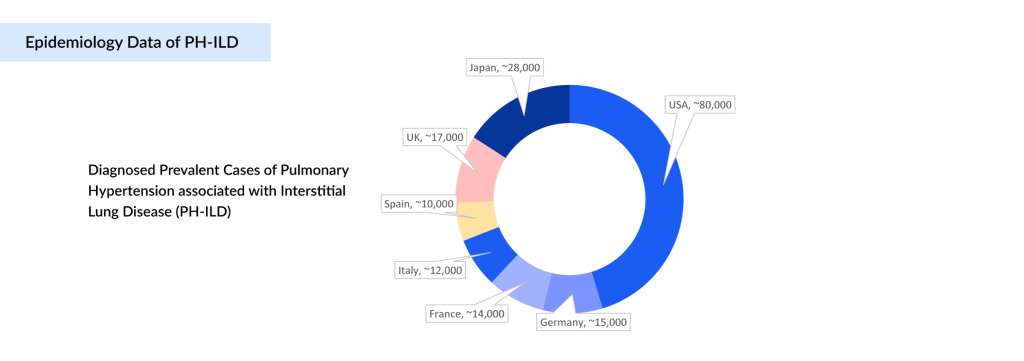
From an epidemiological perspective, PH-ILD is emerging as a significant clinical and public health challenge across major markets, including the United States, EU4 (Germany, France, Italy, Spain), the UK, and Japan. In 2024, DelveInsight estimates indicate there are about 180K PH-ILD diagnosed prevalent cases across these regions, with the burden expected to increase at a notable CAGR through 2034. This anticipated rise reflects multiple factors: heightened disease awareness among clinicians, earlier identification of at-risk patients, and broader use of advanced diagnostic modalities such as High-resolution Computed Tomography (HRCT) and echocardiography.
According to DelveInsight’s analysis, the US accounts for the largest share of the diagnosed population (approximately 45%), followed by the EU4 (Germany, France, Italy, Spain) and the UK markets at around 39%, and Japan at 16%. This distribution aligns with the underlying prevalence of fibrosing ILDs in these regions. The steady upward trend underscores a significant and growing need for therapies that target the unique vascular pathology of PH-ILD, reinforcing the rationale for continued innovation and investment in this space.
Pathophysiological Challenges and Therapeutic Limitations of Pulmonary Hypertension Associated with Interstitial Lung Disease (PH-ILD)
The pathophysiology of PH-ILD is complex and multifactorial. Progressive pulmonary fibrosis disrupts and obliterates the pulmonary vascular bed, while chronic hypoxic vasoconstriction and structural vascular remodeling further elevate pulmonary arterial resistance and pressure. This combination places an increasing workload on the right ventricle, leading over time to right heart dysfunction and, ultimately, right heart failure. Historically, treatments approved for pulmonary arterial hypertension (PAH) have been used off-label in PH-ILD with variable results, reflecting key differences in the underlying disease mechanisms between these patient populations.
Among targeted therapies, prostacyclin analogs, particularly treprostinil, have become the cornerstone for PH-ILD management due to their vasodilatory, antiproliferative, and antithrombotic properties. The FDA’s approval of TYVASO (inhaled treprostinil) for PH-ILD in April 2021, supported by positive outcomes in the pivotal INCREASE trial, was an important milestone that provided evidence of improved exercise capacity and slower disease progression in this challenging patient group.
Nonetheless, important treatment limitations persist. Existing inhaled prostacyclin formulations often require 4 times dosing throughout the day and can cause local side effects such as cough and throat irritation, which may affect patient adherence. Moreover, their impact on long-term disease outcomes remains modest, as they do not fully address the fibrotic and vascular remodeling processes driving PH-ILD progression. These gaps highlight the clear need for more advanced delivery systems and novel therapies that target broader or complementary disease pathways, and they continue to fuel innovation in this evolving therapeutic landscape.
Pulmonary Hypertension Associated with Interstitial Lung Disease (PH-ILD) Treatment Evolution
Historically, therapeutic options for PH-ILD have been scarce, with only a few approved PH-ILD treatments directly addressing the disease’s vascular component. Management has traditionally depended on conventional prostacyclin analogs, which provide limited benefit for many patients. However, the treatment landscape is evolving rapidly. Recent advances in drug development are expanding possibilities beyond standard prostacyclins to include novel inhaled therapies, innovative delivery platforms, and first-in-class agents targeting alternative pathways such as soluble Guanylate Cyclase (sGC) activation, tyrosine kinase signaling, and Phosphodiesterase-5 (PDE5) inhibition.
This article explores this changing landscape in depth, mapping the transition from established inhaled prostacyclin treatments like Treprostinil (TYVASO/TYVASO DPI/ TREPROST and YUTREPIA) to a growing pipeline of next-generation candidates. Key investigational PH-ILD therapies include treprostinil liposomal formulations (L606) and Treprostinil Palmitil Inhalation Powder (TPIP), both designed to enhance drug delivery and duration; Mosliciguat, an sGC stimulator with a unique vasodilation mechanism; Seralutinib, a multi-targeted kinase inhibitor acting on Platelet-derived Growth Factor Receptor (PDGFR), Colony-stimulating Factor 1 Receptor (CSF1R), and c-KIT pathways involved in pulmonary vascular remodeling; H1614 (HB-1614), is a novel inhaled compound that works as a hyaluronan synthesis inhibitor; and Vardenafi (RT234), an inhaled PDE5 inhibitor aimed at rapid symptom relief. By examining these agents’ unique mechanisms, delivery innovations, and emerging clinical data, this article highlights how they may close persistent gaps in PH-ILD treatment.
PH-ILD Marketed Therapies: The Foundation
Management of pulmonary hypertension in patients with ILD is guided by the clinical context, underlying mechanism of pulmonary hypertension, the severity of pulmonary hypertension, and results of vasoreactivity testing performed during right heart catheterization. To a certain extent, pulmonary hypertension in ILD is treatable, whether one is addressing the pulmonary hypertension itself, the underlying ILD, or both.
The underlying lung disease is optimally treated with supplemental long-term oxygen therapy, diuretics, anticoagulants, and pulmonary rehabilitation. Due to age and comorbidities, only a minority of ILD patients are eligible for lung transplantation. Diuretics can optimize fluid balance, and patients are referred for pulmonary rehabilitation. Below are the FDA-approved drugs for PH-ILD treatment:
TYVASO/TYVASO DPI/TREPROST (treprostinil): United Therapeutics
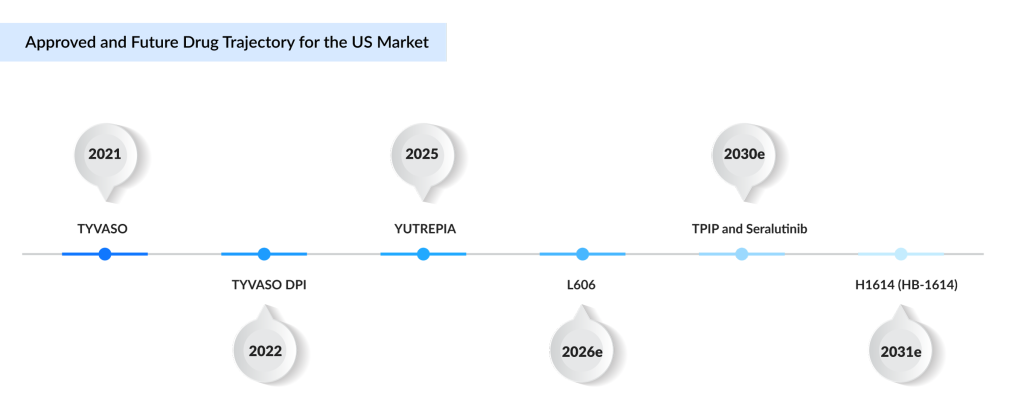
TYVASO (inhaled treprostinil) and its dry powder version, TYVASO DPI, remain the cornerstone therapies for PH-ILD. Originally developed for pulmonary arterial hypertension (PAH, Group 1), these formulations are also approved for PH-ILD (Group 3), helping reduce pulmonary pressures linked to lung scarring and inflammation. The pivotal INCREASE Phase III trial demonstrated that inhaled treprostinil improved Six-minute Walk Distance (6MWD) by an average of 21 m at 16 weeks compared to placebo, with added benefits on NT-proBNP levels and delayed clinical worsening. TYVASO DPI provides a portable, more convenient option than nebulized therapy, improving adherence without altering its mechanism of action. As the first approved treatment for PH-ILD, it has demonstrated strong market uptake. While treprostinil is marketed in Japan as TREPROST (approved September 2024), it has been withdrawn from EU4 and the UK. United Therapeutics also settled patent litigation in 2018 with Watson Laboratories, enabling a generic US launch in 2026; however, core patents extend protection until 2042.
YUTREPIA (treprostinil): Liquidia Corporation
YUTREPIA is a dry powder inhalation formulation of treprostinil sodium, developed with Liquidia’s proprietary PRINT technology to produce precisely engineered particles for targeted deep-lung delivery. Each single-use capsule combines treprostinil with selected excipients to optimize stability and deposition, administered via a convenient, handheld, breath-actuated inhaler. Liquidia submitted YUTREPIA’s New Drug Application (NDA) through the 505(b)(2) pathway, referencing nebulized TYVASO as the listed drug to support bioequivalence. After receiving tentative FDA approval in August 2024, YUTREPIA secured full approval in May 2025 for improving exercise capacity in adults with PAH and PH-ILD. Phase III data confirmed bioequivalence with added usability and adherence benefits. Liquidia launched YUTREPIA commercially in June 2025, just days post-approval, following a decisive court ruling that rejected United Therapeutics’ attempt to block its launch. This underscores Liquidia’s strong legal and regulatory position. While YUTREPIA broadens inhaled prostacyclin options, its mechanism remains within the established vasodilatory and anti-remodeling prostacyclin pathway.
Next-generation Prostacyclin Innovations: Pushing the Delivery Envelope
Some of the PH-ILD drugs in the pipeline include L606 (liposomal treprostinil), Treprostinil Palmitil Inhalation Powder, and others. Let’s dive deeper into the detailed assessment of these drugs.
Treprostinil liposomal (L606): Pharmosa BioPharm/Liquidia Corporation
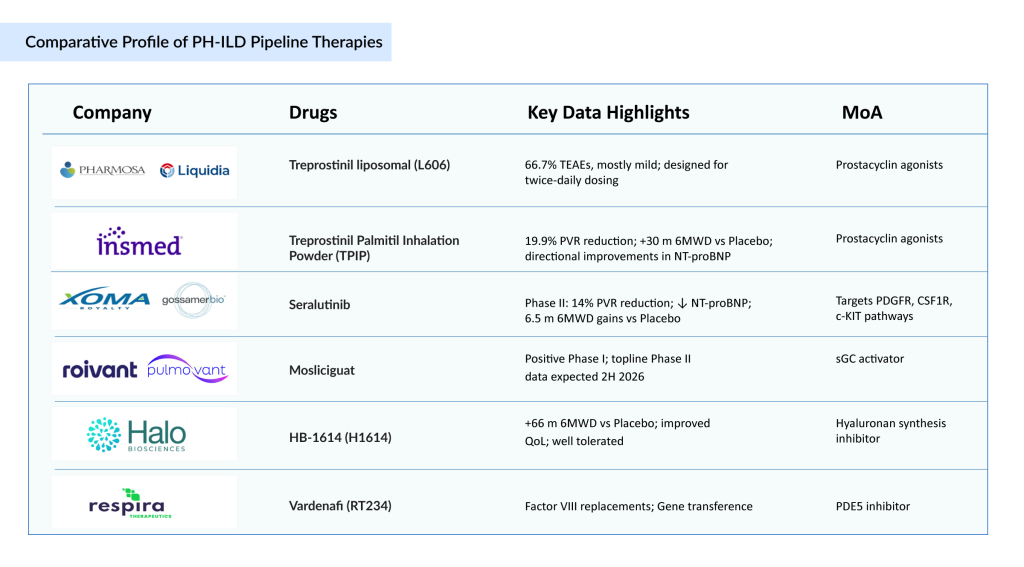
L606 is an inhaled, liposomal treprostinil formulation codeveloped by Pharmosa BioPharm and Liquidia Corporation, now in Phase III trials for PH-ILD. By encapsulating treprostinil in 110–140 nm liposomes within a citrate–bicarbonate buffer, the formulation is designed to sustain pulmonary release, extend drug residence time, and reduce systemic exposure. This could enable twice-daily dosing instead of multiple daily administrations, improving adherence and patient quality of life. Phase III results show a manageable safety profile: 66.7% of patients reported treatment emergent adverse events (TEAEs), mostly mild and unrelated to the drug. Delivered via a next-generation breath-actuated nebulizer, L606 offers rapid, efficient delivery with the potential for better tolerability and consistent pulmonary vasodilation, positioning it as a promising improvement over current therapies.
Treprostinil Palmitil Inhalation Powder (TPIP): Insmed
TPIP is Insmed’s next-generation, once-daily dry powder formulation of a treprostinil prodrug, designed by esterifying treprostinil with palmitic acid to allow for sustained pulmonary release. Administered using a straightforward capsule-based inhaler, TPIP is intended to ease the treatment burden for patients with PAH, PH-ILD, and other serious lung conditions. In studies, TPIP showed a 19.9% placebo-adjusted reduction in pulmonary vascular resistance at 16 weeks and a 30-meter improvement in 6MWD. While there was a trend toward improved NT-proBNP levels from baseline in patients receiving TPIP and a worsening in the placebo group, the difference between the groups was not significant. Insmed plans to launch a Phase III trial in PH-ILD during the second half of 2025.
Beyond Prostacyclins: Novel Mechanisms of Action
Several companies, such as XOMA Royalty, Gossamer Bio, Roivant Sciences, Pulmovant, Halo Biosciences, and Respira Therapeutics, among others, are also working with their lead assets having unique MoA to improve the PH-ILD treatment landscape.
Seralutinib: XOMA Royalty/Gossamer Bio
Seralutinib (GB002) is an investigational inhaled tyrosine kinase inhibitor developed by Gossamer Bio to target PDGFR, CSF1R, and c-KIT—key pathways implicated in vascular remodeling and fibrosis in PAH and PH-ILD. In the Phase II TORREY study for PAH, Seralutinib met its primary endpoint, producing a significant 14% reduction in PVR and lowering NT-proBNP levels. The most pronounced benefits were observed in patients with more advanced disease, although overall gains in six-minute walk distance (6MWD) were 6.5 m. The safety profile was acceptable, with cough being the most frequently reported side effect. Building on these positive results, Gossamer Bio plans to advance Seralutinib directly into a pivotal Phase III trial for PH-ILD, aiming to activate the first clinical sites globally in the fourth quarter of 2025. This strategic approach positions Gossamer Bio to potentially outpace competitors in this underserved space.
Seralutinib’s development path reflects a series of strategic partnerships. Originally licensed by Pulmokine to Gossamer Bio in 2017, Seralutinib became the subject of a global collaboration and license agreement signed in May 2024 between Gossamer Bio and Chiesi Farmaceutici. To further strengthen its rare disease portfolio, XOMA Royalty Corporation acquired Pulmokine, adding Seralutinib as a Phase III–ready asset.
Mosliciguat: Roivant Sciences/Pulmovant
Mosliciguat (BAY 1237592) is a once-daily inhaled sGC activator currently in Phase II development for PH-ILD, with positive Phase I results supporting its advancement. By directly activating sGC independent of nitric oxide (NO), Mosliciguat is well-positioned for front-line use in PH-ILD, where endothelial dysfunction limits NO bioavailability. Topline data from the ongoing Phase II trial in PH-ILD are expected in the second half of 2026. Strong granted patents and pending applications could secure protection until at least 2042, excluding potential Patent Term Extensions (PTE). This robust IP position enhances Pulmovant’s ability, following Roivant’s acquisition of global rights from Bayer in September 2024, to maximize Mosliciguat’s market potential as a first-in-class therapy.
H1614 (HB-1614): Halo Biosciences
HB-1614 (H1614 or H01) is Halo Biosciences’ lead investigational therapy, based on a proprietary reformulation of 4-methylumbelliferone (4-MU), a well-characterized small molecule that selectively inhibits hyaluronan synthesis—a key driver of chronic inflammation and fibrosis within the Extracellular Matrix (ECM). Excessive hyaluronan accumulation is increasingly recognized as a pathological hallmark in progressive fibrotic and vascular diseases. In the Phase II SATURN study involving 17 patients with PH-ILD, H1614 (HB-1614) demonstrated encouraging safety and exploratory efficacy signals, with patients experiencing a mean improvement of 66 m in six-minute walk distance (6MWD) and better quality-of-life scores over 24 weeks. The therapy was well tolerated, supporting its potential as a differentiated, novel oral option for PH-ILD
Vardenafi (RT234): Respira Therapeutics
RT234 is Respira Therapeutics’ innovative inhaled formulation of vardenafil hydrochloride, a Phosphodiesterase type 5 (PDE5) inhibitor traditionally used orally for erectile dysfunction. Unlike chronic oral PAH treatments, RT234 is designed for “as-needed” (pro re nata) use to deliver rapid, targeted pulmonary vasodilation, aiming to relieve exertional dyspnea and enhance exercise capacity in patients with PH-ILD. By leveraging preclinical and Phase I data from RT234-PAH trials conducted in Australia, Respira is advancing RT234 PH-ILD as a Phase II–ready therapy for PH-ILD. Its inhaled route ensures fast lung absorption, with an extended duration of action and minimal overlap with maintenance therapies, providing flexible symptom control and addressing a significant gap for patients needing on-demand relief during physical activity.
The United States PH-ILD Pipeline Outlook
The treatment landscape for PH-ILD in the US is undergoing a measured but strategic evolution, driven by both incremental improvements to established prostacyclin pathways and the emergence of therapies that target novel disease mechanisms. The approvals of TYVASO in April 2021 and TYVASO DPI in June 2022 demonstrated the viability of inhaled treprostinil in improving exercise capacity and delaying clinical worsening, while Liquidia’s YUTREPIA, approved in May 2025, exemplifies how advanced particle engineering can optimize deep-lung delivery and patient convenience. Looking ahead, DelveInsight estimated that Treprostinil liposomal (L606) could reach the US market by 2026, potentially offering sustained drug release and fewer daily doses—addressing long-standing adherence challenges. By 2030, TPIP’s once-daily prodrug design and Seralutinib’s multi-kinase inhibition of key remodeling pathways could expand the therapeutic scope beyond pure vasodilation to directly target fibrosis and vascular proliferation. HB-1614 (H1614), projected for a 2031 launch, represents an entirely different approach as an oral hyaluronan synthesis inhibitor tackling ECM-driven fibrotic pathways. These assumptions, grounded in late-stage efficacy and safety data, Primary Completion Dates (PCD), and development milestones, highlight how the next decade could see PH-ILD shift from single-mechanism maintenance to more individualized, mechanism-driven treatment strategies.
Conclusion: Redefining the Future of the PH-ILD Treatment Landscape
The evolving PH-ILD pipeline signals a paradigm shift from symptom-focused vasodilation toward a precision approach that tackles the disease’s multifactorial roots—fibrosis, inflammation, and vascular remodeling. While established prostacyclins like TYVASO and YUTREPIA validate the importance of inhaled delivery for functional gains, next-generation formulations such as Treprostinil liposomal (L606) and Treprostinil Palmitil Inhalation Powder (TPIP) aim to address adherence barriers through sustained and simplified dosing. Critically, emerging candidates like Seralutinib, Mosliciguat, Vardenafil (RT234), and H1614 (HB-1614) exemplify how targeting distinct molecular pathways, multi-kinase signaling, sGC activation, PDE5 inhibitors, and hyaluronan synthesis could deliver additive or synergistic benefits beyond vasodilation alone. This mechanistic diversification aligns with growing epidemiological awareness and the push for earlier diagnosis, creating a fertile environment for stratified, patient-specific treatment algorithms. As these novel agents advance, real-world outcomes will determine whether they can meaningfully alter long-term right heart function and survival. If successful, the next decade could redefine PH-ILD from a historically neglected complication to a model of innovation-driven chronic disease management.

Downloads
Article in PDF