Small Cell Lung Cancer Market Booms with an Influx of Companies and Robust Pipeline
Jul 03, 2020
As per DelveInsight, the Small cell lung cancer market size is expected to increase at a CAGR of 15.2% during the study period (2017–30) attributing to a rich pipeline, healthcare spending, improved R&D, and discovery of novel targets.
Small cell lung cancer (SCLC) or Small cell lung carcinoma is an aggressive and most malignant form of tumor, characterized by uncontrolled, rapid growth of certain cells in the lungs. It accounts for almost 15% of all the lung cancers, the deadliest form of cancer in the world. Due to the belief of their origin from neuroendocrine cells (APUD cells) in the bronchus, SCLC is categorized as a type of neuroendocrine tumor of the lungs. According to a study conducted by Mitchell, Richard Sheppard, et al., small cell lung cancer produces a variety of neuroendocrine markers that can render the patient at risk of developing Paraneoplastic syndromes and Cushing’s syndrome due to the production of hormones like ADH and ACTH. The fact was very well supported by another research by Verschuuren JJ, et al. which confirmed that at least 60% of the Lambert-Eaton myasthenic syndrome (LEMS) patients have an underlying malignancy usually of Small cell lung cancer.
DelveInsight estimated that approximately 25% of all the cases were of limited-stage whereas the remaining 75% of the cases were observed to be of Extensive-stage SCLC, in the 7MM (the US, EU5 (the UK, Germany, France, Italy and Spain), and Japan) in 2017. Moreover, total Small-cell lung cancer incident cases are expected to increase with a CAGR of 1.7% for the study period 2017-30. Furthermore, the US accounted for the highest SCLC incidence in the 7MM, with Germany leading among EU-5 countries.
Downloads
Click Here To Get the Article in PDF
Small cell lung cancer appears across all the age group; however, middle-aged and older adults constitute most of the SCLC patient pool. Interestingly enough, a gender gap can be witnessed with a higher male SCLC incidence than females. As per DelveInsight, approximately 55% of the diagnosed pool consisted of males; however, the incidence gap has started to get narrower in recent years. The trend can be attributable to the rise in smoking, which is a significant risk factor of Small cell lung cancer.
Small cell lung cancer is an aggressive tumor and has earlier development of metastases. Based on the process of staging, they are categorized into Limited-stage and Extensive-stage Small cell lung cancers. Limited-stage SCLC is often confined to one side of the lung. Cancer in supraclavicular nodes can also be categorized as Limited-stage if confined to one side of the chest; however, if cancer spreads beyond one side of the chest to other, to both the lungs and farther beyond in bone marrow and other body parts, it is referred to as Extensive-stage Small cell lung cancer. It has been observed that about three-quarters of SCLC patients are diagnosed with extensive-stage disease. While, Limited stage small cell lung cancers, due to their confinement to a single side of the chest, can be treated with ease with a single radiation field, in the case of extensive-stage SCLC, individuals are administered with a combination of surgeries, chemotherapies, radiation therapies and targeted therapies.
There are a number of treatment options for Extensive-Stage Small cell lung cancer (ES-SCLC), including surgery, chemotherapy, radiation therapy and immunotherapy, or a combination of the therapies depending upon the stage, patient’s health and severity of the tumor. Past years, the Small cell lung cancer market witnessed several approvals for the treatment of metastasized SCLC. Along with chemotherapies, of which the most commonly used are Etoposide or Irinotecan, plus a platinum-based drug such as Cisplatin or Carboplatin, there has been a shift towards personalized treatments tailored to suit individuals.
Nivolumab (Opdivo) by Bristol-Myers Squibb and Pembrolizumab (Keytruda), a gene therapy candidate of Merck, both are PD-1 inhibitors. PD-1 is a protein on T-cells that ceases the ability of T-cell from attacking other cells. The gene therapies block the protein harnessing the immune cell’s own power to kill the cancerous cells. In addition to Nivolumab and Keutruda, Atezolizumab (Tecentriq), a PD-L1 inhibitor of Genentech, received FDA approval for the first-line treatment of adult patients with extensive-stage SCLC in combination with carboplatin and Etoposide.
Even with the existing therapies in the Small cell lung cancer market, there appear significant unmet needs, and there are many areas of treatment in which pipeline products can improve upon existing therapies. Not to be missed that both Nivolumab and Pembrolizumab are not authorized for the treatment of SCLC in the European Region as well as in Japan. Considering the complexities of the tumor, and rapid progression from the lungs to other parts that lead to poor prognosis eventually causing deaths of individuals, the current Small cell lung cancer market calls for an increase in the range of targeted therapies, a decrease in the toxicity of chemotherapies, and a general improvement in prognosis.
Small Cell Lung Cancer Market: Market Opportunities and Hurdles
The therapeutic option for patients with relapsed-SCLC is minimal. However, several clinical trials are underway, including the trials studying the efficiency and safety of Nivolumab in several trials (NCT03043599, NCT03670056, NCT02538666, NCT02481830, and NCT03083691). However, recent announcements by the company regarding the failure of the drug in meeting primary endpoints in two of Phase III trials, NCT02538666, and NCT02481830, with no significant improvement in OSS when compared to chemotherapy, proved to be a setback for growing Small cell lung cancer pipeline. Furthermore, a phase II trial that was focused on studying a combination therapy of Nivolumab and Ipilimumab, demonstrated that although the duo managed to improve Overall Response Rate in patients with high Tumor mutation burden, however, at the cost of high toxicities thus there is a need of balancing risks and treatment benefit.
Merck is also conducting several studies evaluating the Pembrolizumab alone and in combination with Cisplatin, Carboplatin, Etoposide, and Amurubicin (NCT03066778, NCT02934503, NCT03253068, NCT03066778, and NCT02580994). However, the path did not seem to be smooth as the company faced a snag after one of its regimen, a duo of Pembrolizumab and chemotherapy, in the pipeline for the first-line treatment for patients with ES-SCLC failed to improve OSS rates by significant per cent.
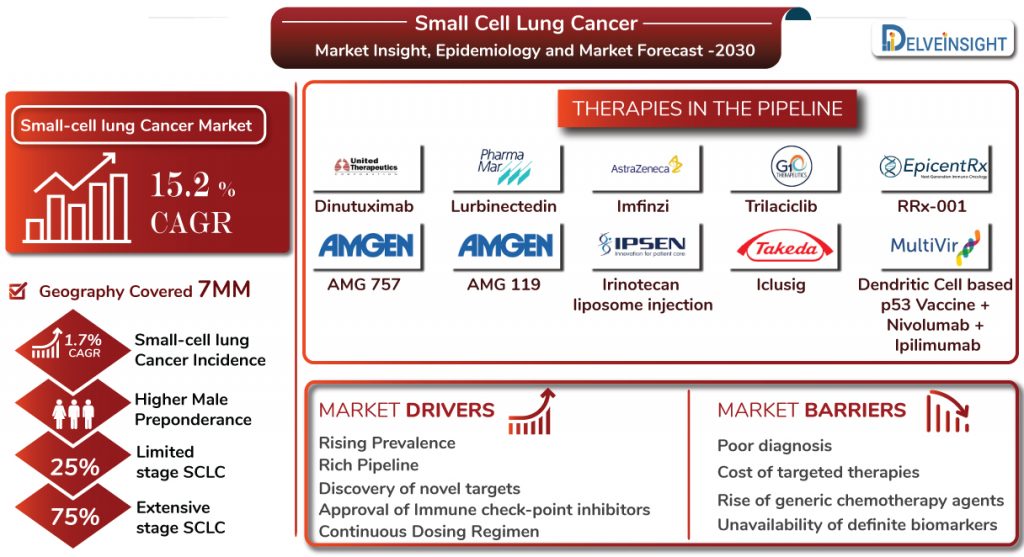
The unmet needs and untapped opportunities have caught the attention of several pharma players such as AstraZeneca (Imfinzi), G1 Therapeutics (Trilaciclib), PharmaMar, (Lurbinectedin; Zepsyre, PM1183), United Therapeutics (Dinutuximab), EpicentRx (RRx-001), and others. The active participation of the companies has resulted in active ES-SCLC pipeline, assuming most of the patients opting for therapies other than radiation therapy are suffering from Extensive-stage Small cell lung cancer. Therefore, emerging drugs in the SCLC pipeline are mainly targeting extensive-stage SCLC patients in clinical trials. Additionally, companies including Amgen and Fate Therapeutics are also investing their time, energy and funds in improving the Small cell lung cancer market with their therapies still at early stages of development. Amgen has two of its therapies in its armamentarium, AMG 757 and AMG 119, under evaluation for relapsed SCLC.
What’s in the Horizon
The dynamics of the ES-SCLC market is anticipated to change in the coming years owing to the improvement in the rise in healthcare spending across the world. However, there are challenges that exist in the form of unavailability of definite biomarkers available for progressive or the transformative form of the disease that calls for an increase in R&D in the field. Timely diagnosis of tumor can help in leveraging the treatment regimen preventing the risk of eventual deaths, however, for this proper diagnosis modalities and tumor screening methodologies are needed. Moreover, with an inclination towards precision medicine, the cost of gene therapies and a high out-of-pocket expenditure always have haunted the patients, for which healthcare authorities restrict pricing and usage of high-cost agents that offer relatively low or moderate efficacy or no added benefit compared to current treatments on the price they are offered. Recently, NICE (National Institute for Clinical Excellence) rejected Roche’s Tecentriq in new lung cancer use, suggesting the company to drop its therapy price to gain regular NHS funding in this indication.
Overall, the Small cell lung cancer market size growth, if obstructed by barriers, is also fuelled by market drivers. With a robust SCLC pipeline, the dynamics of the ES-SCLC market is anticipated to change in the coming years owing to healthcare spending across the world. As per DelveInsight’s estimation, the Small cell lung cancer market size is expected to increase at a CAGR of 15.2% during the study period (2017–2030). Taking into account the emerging therapies that have novel targets, entry of fast-check point inhibitors such as with better clinical profile such as PD-1 inhibitor, CDK4/6 Inhibitor and RNA polymerase II inhibitors, which are expected to further dominate the SCLC market for the forecast period 2020-30 as well as proper treatment algorithm for the patients with continuous dosing regimens, the Extensive-Stage Small cell lung cancer market in the 7MM is expected to increase by 2030. Moreover, SCLC pipeline drugs have the benefit to be labelled as orphan drugs. They are given accelerated approvals, seven years of market exclusivity, clinical trials subsidies and reduced regulatory fees, which further attract a significant number of companies to explore Small cell lung cancer market.
Total Small-cell lung cancer incident cases are expected to increase with a CAGR of 1.7% for the study period 2017-30 in the 7MM.
As per DelveInsight’s estimation, the Small cell lung cancer market size is expected to increase at a CAGR of 15.2% during the study period (2017–2030).
Key pharma players such as AstraZeneca, G1 Therapeutics, PharmaMar, United Therapeutics, EpicentRx, Amgen and Fate Therapeutics and others are advancing the Small cell lung cancer market.
Unavailability of definite biomarkers, lack of proper diagnosis methodologies, targeted therapies with a balance between treatment and toxicities, price of gene therapies, and others are major unmet needs in the SCLC market.
Dinutuximab, Lurbinectedin, Imfinzi, Trilaciclib, RRx-001, AMG 757, AMG 119 and others are the therapies in the Small cell lung cancer pipeline.
Downloads
Article in PDF
Recent Articles
- Tetra, Shionogi sign deal; Amgen remunerates; Gilead’s fibrotic disease deal
- Business Cocktail
- Potential Diagnostic solutions that may significantly contribute to COVID-19 detection
- Letter for AstraZeneca; Patheon on API plant; Sanofi, Regeneron’s Dupixent; Cerulean Pharma and D...
- ImaginAb joins hands with AstraZeneca, Pfizer, Takeda



