Biogen
Feb 12, 2025
Challenges in the Pursuit of Alzheimer’s Disease Treatment Breakthroughs: Drug Clinical Trial Failures
Alzheimer’s, the most common type of dementia, particularly with an increasing geriatric population, presents a major global crisis. The disease mostly occurs in sexagenarians, but it may manifest in quadragenarians. It is one of the most prevalent neurodegenerative disorders with a multifactorial pathogenesis. It ...
Read More...
Feb 27, 2024
Survodutide Phase II trial Shows Groundbreaking Results in Liver Disease; GSK Announces Positive Headline Results from EAGLE-1 Phase III Trial; Dupixent sBLA Accepted for FDA Priority Review; Biogen’s QALSODY Received Positive Opinion from CHMP; FDA Granted Orphan Drug Designation to Immune-Onc’s IO-202; Artiva Biotherapeutics’s AlloNK® in Lupus Nephritis
Survodutide Phase II trial Shows Groundbreaking Results in Liver Disease due to MASH, with Significant Improvements in Fibrosis Boehringer Ingelheim has reported that in a Phase II trial, a significant proportion of adults treated with survodutide (BI 456906), up to 83.0%, showed a notable enhancement in metabol...
Read More...
Feb 13, 2024
GSK Receives FDA Fast Track Designation for Bepirovirsen; Gilead to Acquire CymaBay Therapeutics; CSL Announces Top-line Results from the Phase III AEGIS-II Trial; Ruxoprubart Scores FDA Orphan Drug Designation for PNH Treatment; CymaBay Announces FDA Acceptance of NDA and Priority Review for Seladelpar; Biogen Received European Commission Approval for SKYCLARYS
GSK Receives FDA Fast Track Designation for Bepirovirsen in Chronic Hepatitis B GSK plc has revealed that the US Food and Drug Administration (FDA) has awarded Fast Track status to bepirovirsen, an experimental antisense oligonucleotide (ASO) designed to treat chronic hepatitis B (CHB). Fast Track designation ai...
Read More...
Dec 19, 2023
Eisai Submits Marketing Authorization Application for Tasurgratinib; CHMP Issues Positive Opinion for Biogen’s SKYCLARYS; European Commission Approves Merck’s KEYTRUDA + Chemotherapy HER2-ve Gastric or GEJ Adenocarcinoma; BMS Provides Update on RELATIVITY-123 Trial; Kyverna Therapeutics Granted FDA Fast Track Designation for KYV-101; Verrica and Torii Pharma Announces Positive Top-line Results from a Confirmatory Phase 3 Trial of TO-208
Eisai Submits Marketing Authorization Application In Japan for Anticancer Agent Tasurgratinib For Biliary Tract Cancer With FGFR2 Gene Fusion Eisai Co., Ltd. has officially submitted a request for marketing authorization in Japan for tasurgratinib succinate, its internally developed tyrosine kinase inhibitor tar...
Read More...
Sep 26, 2023
LEQEMBI Intravenous Infusion Approval; Novartis’ Presented Updates on Lutathera; FDA Accepts Submission to Add PH-ILD to YUTREPIA Label; FDA Issues CRL to BLA for Pegfilgrastim-cbqv; FDA Fast Track Designation to Therpay, MWTX-003; EC Approves TEPKINLY (epcoritamab) for R/R DLBCL
Disc Medicine Receives FDA Fast Track Designation for MWTX-003 for the Treatment of Polycythemia Vera On Sept. 20, 2023, Disc Medicine, Inc. (NASDAQ: IRON) announced that the United States Food and Drug Administration (FDA) has granted Fast Track Designation to MWTX-003 for the treatment of patients with Polycyt...
Read More...
Aug 21, 2023
Mixed Fortunes for Biogen and Sage’ Zuranolone: Approval Under the Cloud of Rejection
In a bittersweet decision for Biogen and Sage Therapeutics, the FDA approved the fast-acting drug zuranolone as the first tablet for postpartum depression on 04 August —but rejected the drug for major depressive disorder treatment. Zurzuvae is a neuroactive steroid that acts as a GABA-A receptor-positive allosteric...
Read More...
Aug 08, 2023
FDA Approves ZURZUVAE for Postpartum Depression; Astellas Drug Acquired in $5.9B Deal Wins FDA Approval; FDA Clearance to Phase III Study of Lisaftoclax; FDA Issues CRL to BLA Resubmission for Remestemcel-L; Bavarian Nordic Updated on its Chikungunya Virus Vaccine; FDA Orphan Drug Designation to ABM-1310
FDA Approves ZURZUVAE, the First and Only Oral Treatment Approved for Women with Postpartum Depression Biogen Inc. and Sage Therapeutics, Inc. announced that the FDA has approved ZURZUVAE (zuranolone) 50 mg for individuals with postpartum depression (PPD). ZURZUVAE is the first and only 14-day oral, once-daily t...
Read More...
Aug 01, 2023
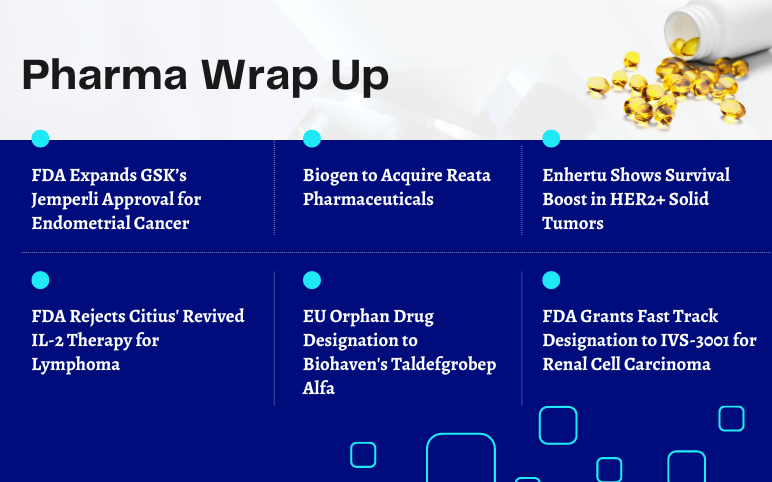
FDA Expands GSK’s Jemperli Approval; Biogen to Acquire Reata Pharma; Enhertu Shows Survival Boost in HER2+ Solid Tumors; FDA Rejects Citius’ Revived IL-2 Therapy; FDA Fast Track designation to IVS-3001 for RCC; EU Orphan Drug Designation to Biohaven’s Taldefgrobep Alfa
FDA Expands GSK’s Jemperli Approval for Endometrial Cancer GSK plc announced that the US Food and Drug Administration (FDA) has approved Jemperli (dostarlimab) in combination with carboplatin and paclitaxel, followed by Jemperli as a single agent for the treatment of adult patients with mismatch repair deficient...
Read More...
Jul 11, 2023
FDA Grants Priority Review for Zolbetuximab BLA; FDA Traditional Approval for LEQEMBI for Alzheimer’s Disease; Iovance Announces Regulatory and Clinical Updates for TIL Therapy in Advanced NSCLC; Biophytis Seeks FDA Approval to Launch Phase 3 Study of Potential Treatment of Sarcopenia; Orphan Drug Designation to Marker Therapeutics’s MT-401 for AML Treatment; Axsome Therapeutics Initiates Phase 3 Trial of Solriamfetol for ADHD
Astellas Announces FDA Grants Priority Review for Zolbetuximab Biologics License Application Astellas Pharma Inc. announced that the FDA has accepted and granted Priority Review for the company's Biologics Licence Application (BLA) for zolbetuximab, a first-in-class investigational Claudin 18.2 (CLDN18.2)-target...
Read More...
May 02, 2023
FDA Grants Priority Review to BMS’ Luspatercept; Teva and MedinCell’s Risperidone FDA Approval; Biogens’s QALSODY FDA Accelerated Approval; FDA IND Authorization to Kiromic’s Deltacel; Atsena’s ATSN-201 FDA IND Clearance
FDA Grants Priority Review to Luspatercept for First-line Treatment of Anemia in Lower-risk MDS The FDA has granted priority review to a supplemental biologics license application (sBLA) seeking to expand the current indication of luspatercept-aamt (Reblozyl) to include treatment of anemia in patients with very ...
Read More...







-Agonist.png)

