bladder cancer
Apr 01, 2025
Sanofi’s Qfitlia Becomes First FDA-Approved Therapy for Hemophilia A or B; FDA Approves AstraZeneca’s IMFINZI as First Perioperative Immunotherapy for Bladder Cancer; Beam Therapeutics’ BEAM-302 for AATD Receives FDA Clearance; AstraZeneca’s CALQUENCE Combo Greenlit in EU for Mantle Cell Lymphoma; Ionis’ WAINZUA Wins EU Approval for Hereditary Transthyretin Amyloidosis
Sanofi’s Qfitlia Becomes First US-Approved Therapy for Hemophilia A or B The FDA has approved Qfitlia (fitusiran), the first antithrombin-lowering therapy for routine prophylaxis to prevent or reduce bleeding episodes in hemophilia A or hemophilia B patients (ages 12+) with or without inhibitors. Approval is bas...
Read More...
Aug 26, 2024
Imbalance Between BCG Supply and Increased Demand: The Crisis Impacting Bladder Cancer Treatment
The BCG vaccine, a significant medical breakthrough developed in the early 20th century, is derived from a strain of Mycobacterium bovis initially created by the University of Illinois as a vaccine against tuberculosis. For NMIBC, BCG is administered intravesically to reduce the risk of cancer recurrence and progre...
Read More...
Feb 06, 2024
4DMT Presents Data From Phase II PRISM Clinical Trial; Adaptimmune Announces FDA Acceptance of Biologics License Application for Afami-cel; Astellas Submits Supplemental New Drug Application in Japan for PADCEV with KEYTRUDA; AnaMar Announces US and EU Orphan Drug Designation for AM1476; Biosyngen Announces FDA Fast Track Designation for BST02; Acepodia Announces FDA Clearance of IND Application for ACE2016
4DMT Presents Positive Interim Data from Randomized Phase II PRISM Clinical Trial of Intravitreal 4D-150 Demonstrating Favorable Tolerability & Clinical Activity in Wet AMD 4D Molecular Therapeutics, a prominent company in the field of genetic medicines with a focus on harnessing the full potential of geneti...
Read More...
Apr 11, 2023
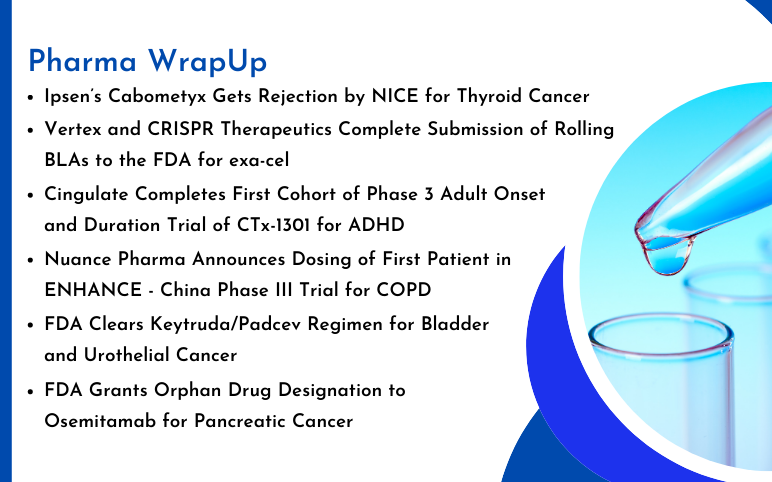
Ipsen’s Cabometyx Rejected by NICE; Vertex and CRISPR Therapeutics’s Submit BLA to the FDA for exa-cel; Orphan Drug Designation to Osemitamab for Pancreatic Cancer; FDA Clears Keytruda/Padcev for Bladder and Urothelial Cancer; Cingulate Completes Trial of CTx-1301 for ADHD; Nuance Pharma Announces Dosing of First Patient in ENHANCE Trial
FDA Grants Orphan Drug Designation to Osemitamab for Pancreatic Cancer Transcenta Holding Limited has announced that the U.S. Food and Drug Administration (FDA) has awarded Orphan Drug Designation to Osemitamab (TST001), a highly potent humanized monoclonal antibody that enhances ADCC (antibody-dependent cell-me...
Read More...
Jul 12, 2022
Novo Nordisk’s Concizumab for Hemophilia; AbbVie Ends its Alliance with Alector; ADC Therapeutics and Sobi Enters in Exclusive Licensing Deal; BMS’ Opdivo Gets NHS Use; Merck to Acquire Seagen; FDA Priority Review to Roche’s Lunsumio; AstraZeneca to Acquire TeneoTwo; FDA Orphan Drug Designation to PBI-200
Novo Nordisk Reports Phase III Results of Concizumab Drug for Hemophilia A or B Novo Nordisk has announced Phase III results for their concizumab drug for hemophilia A or B, demonstrating efficacy in preventing bleeding events and paving the way for regulatory filings later this year. Concizumab, an anti-tissue ...
Read More...
Jun 07, 2022
CG Oncology Teams Up With Merck & BMS; Parse Biosciences Partners With Molecular Diagnostics Korea; Amgen’s Olpasiran; Elevar Therapeutics Announces Results of Rivoceranib; FDA Approves GSK’s Measles Vaccine; Sage & Biogen Reveals Postpartum Depression Trial Result
FDA Gives Green Signal to First Measles Vaccine in Almost 50 Years GSK announced that the company's vaccine for protection against mumps, measles, and rubella (MMR) had been approved by the U.S. FDA. According to data from six clinical studies, the company filed a Biologics License Application in August 2021. ...
Read More...
Feb 25, 2022
Seagen/Astellas plans to expand their Antibody Drug Conjugate (ADC), Padcev in additional bladder cancer settings?
Padcev (Enfortumab vedotin) for the treatment of locally advanced or metastatic urothelial cancer, Adcetris (Brentuximab vedotin) for the treatment of several types of CD30-expressing lymphomas, and Tivdak (Tisotumab vedotin) for the treatment of recurrent or metastatic cervical cancer are the three ADCs that Seage...
Read More...
Oct 03, 2019
Roche’s Tecentriq combo proves effective in Bladder cancer
Roche’s Genentech has presented positive Phase III results from the IMvigor130 study aimed at studying Tecentriq (atezolizumab) tested in combination with platinum-based chemotherapy. The drug, Tecentriq, combined with Pt-based chemotherapy showed a noteworthy improvement in patients with untreated advanced bla...
Read More...
Apr 16, 2019
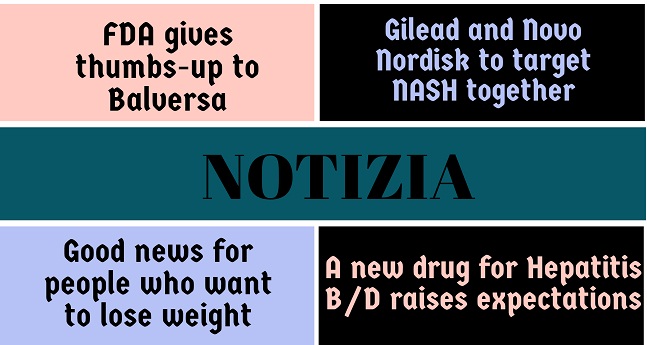
Notizia
FDA gives thumbs-up to Balversa The U.S. FDA has granted approval of Balversa (erdafitinib) to Belgium-based Janssen Pharmaceutica, for the treatment of advanced or metastatic bladder cancer. This drug is the first ever approved target-specific medication targeting susceptible FGFR genetic mutations, and for can...
Read More...
Oct 31, 2018
The risk of solid cancer after chemotherapy
Subsequent malignant neoplasms (SMNs) are one of the most serious and potentially lethal complications of cancer and its therapy. Radiotherapy has been known to be a human carcinogen, however, a growing body bolsters the selective contribution of chemotherapeutic agents to SMN risk. Cisplatin binds to and destructs ...
Read More...






-Agonist.png)

