cancer diagnostics devices
Sep 10, 2025
From Lab to Bedside: The Rise of AI-Enabled Digital Pathology
Pathology—the discipline of diagnosing disease through tissue analysis, has long depended on glass slides and microscopes. Today, digital pathology is undergoing a remarkable shift, as whole-slide imaging (WSI) and artificial intelligence (AI) come together to streamline lab workflows, accelerate diagnoses, and enh...
Read More...
May 09, 2024
Monteris Smallest Brain Laser Probe Launch; Vesica Health’s AssureMDx Test Launch; Anaut’s Eureka α Japanese Regulatory Approval; Geneoscopy’s Labcorp-Partnered Colon Cancer Test FDA Approval; Novel Omeza® OCM™ Diabetic Foot Ulcers Results; LEO Pharma Phase III Plaque Psoriasis Trial Results
Monteris Launched Smallest Brain Laser Probe on the Market On May 01, 2024, Monteris Medical launched the NeuroBlate® NB3™ FullFire® 1.6mm laser probe, the company’s latest product line innovation for use with their market-leading NeuroBlate System. The NB3 laser probe, which integrates Monteris' patented coo...
Read More...
Jan 18, 2024
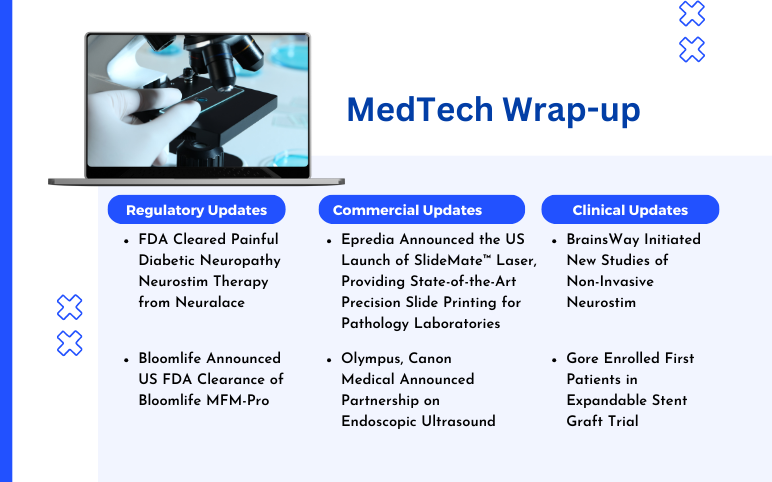
Epredia Announced the US Launch of SlideMate Laser; Olympus, Canon Medical Announced Partnership; FDA Cleared Painful Diabetic Neuropathy Neurostim Therapy from Neuralace; FDA Clearance to Bloomlife’s Bloomlife MFM-Pro; BrainsWay Initiated New Studies of Non-Invasive Neurostim; Gore Enrolled First Patients in Expandable Stent Graft Trial
Epredia Announced the US Launch of SlideMate™ Laser, Providing State-of-the-Art Precision Slide Printing for Pathology Laboratories On January 11, 2024, Epredia, a global leader in precision cancer diagnostics, announced that the company launched US sales of SlideMate™ Laser, the newest addition to the company’s...
Read More...
Jul 07, 2022
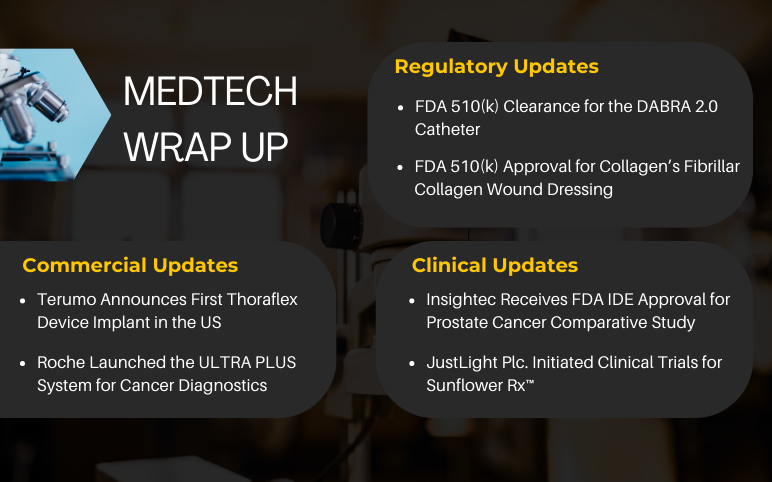
Collagen Matrix FDA 510(k) approval for Fibrillar Collagen Wound Dressing; Roche’s cancer diagnostics ULTRA PLUS system launch; Insightec IDE Approval for Prostate Cancer; JustLight plc. trial of Sunflower Rx for Alzheimer’s disease; Thoraflex Hybrid Device Implantation in the United States; FDA 510(k) Clearance for the DABRA 2.0 Catheter
Collagen Matrix received FDA 510(k) approval for Fibrillar Collagen Wound Dressing On June 29, 2022, Collagen Matrix, Inc., a leader in regenerative medicine, a global manufacturer of collagen and other biomaterial-based medical devices, and Linden Capital Partners portfolio company announced FDA 510(k) Clearan...
Read More...


-Agonist.png)

