Left Atrial Appendage Closure Devices Market
Nov 21, 2024
Cartessa Aesthetics teams with Classys for Everesse; LogicMark unveils Freedom Alert Max; GE HealthCare clears SIGNA MAGNUS MRI; OMRON’s BP monitor with AI-powered AFib detection gets FDA approval; Boston Scientific’s WATCHMAN FLX™ cuts bleeding risk; Encora Therapeutics wraps ULTRE trial for tremor device
Cartessa Aesthetics Partnered With Classys, Inc. to Bring Everesse to the US Market On November 14, 2024, Cartessa Aesthetics, a leading North American aesthetic medical device company, collaborated with Classys, Inc., a prominent global aesthetics firm based in South Korea, to introduce EVERESSE by Volnew...
Read More...
Sep 07, 2023
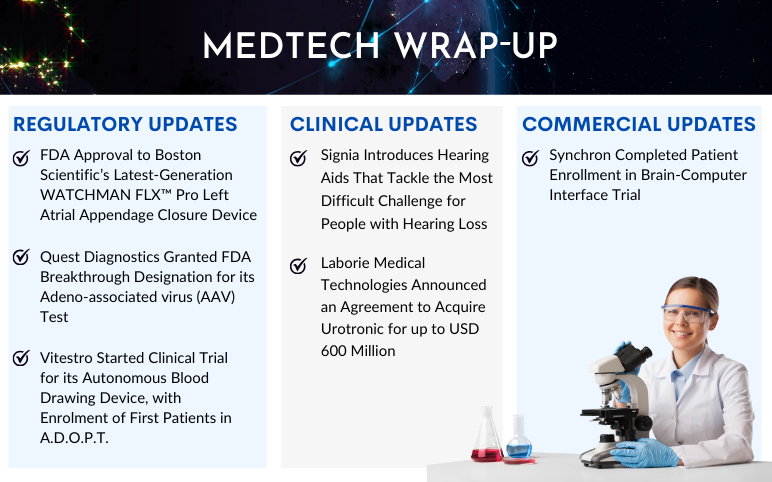
Boston Scientific’s WATCHMAN FLX™ Pro; Quest Diagnostics’s AAV Test; Vitestro Started A.D.O.P.T. Clinical Trial; Signia Introduces Hearing Aids; Laborie Medical to Acquire Urotronic; Synchron’s Brain-Computer Interface Trial
Quest Diagnostics Granted FDA Breakthrough Designation for its Adeno-associated virus (AAV) Test On August 30, 2023, Quest Diagnostics announced that its AAVrh74 ELISA assay (CDx) has been granted Breakthrough Device Designation from the US Food and Drug Administration (FDA). The enzyme-linked immunosor...
Read More...
Feb 02, 2023
Alma Announced the Launch of Alma Duo; Phillips-Medisize Launched a Pen Injector Platform; FDA Approved Abbott’s Spinal Cord Stimulation; Axonics Received FDA Approval for Rechargeable Sacral Neuromodulation; SELUTION SLR Coronary Sirolimus DEB Study Enrolls First Patient; AtriCure LeAAPS Clinical Trial
Alma announced the Launch of Alma Duo- an Advanced Solution for Sexual Wellness On January 26, 2023, Alma, the core subsidiary of Sisram Medical and one of the leaders of energy-based medical and aesthetics solutions, launched its new sexual wellness product, Alma Duo. Alma Duo is a cutting-edge, safe, non-in...
Read More...
Mar 10, 2022
Bioventus’s StimRouter Pain Management Device; FDA Approval for eCoin Therapy; SurGenTec Launches ION Screw; Exactech’s Humeral Reconstruction Prosthesis; Boston Scientific’s WATCHMAN FLX LAAC Device; PathMaker Neuro’s MyoRegulator
Bioventus Receives 501(k) Clearance for StimRouter Pain Management Device On March 1, 2022, the US Food and Drug Administration (FDA) gave Bioventus' StimRouter Neuromodulation System 510(k) approval. The next-generation pain treatment device is intended to treat chronic pain caused by peripheral nerves, ex...
Read More...


-Agonist.png)

