medical device
Sep 07, 2023
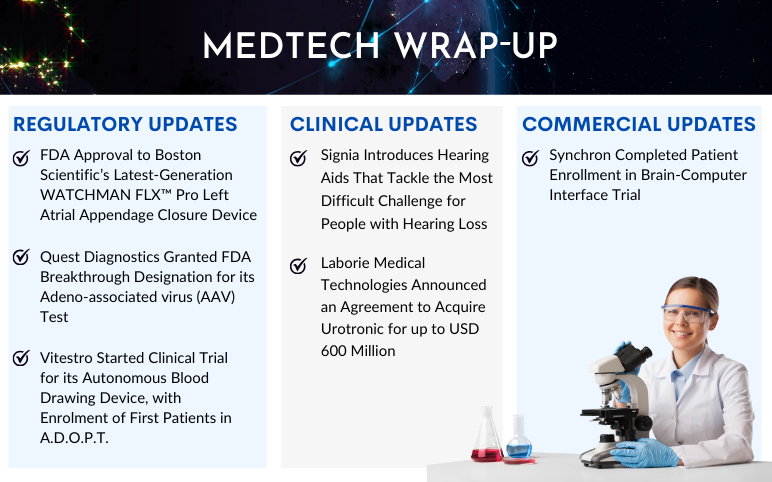
Boston Scientific’s WATCHMAN FLX™ Pro; Quest Diagnostics’s AAV Test; Vitestro Started A.D.O.P.T. Clinical Trial; Signia Introduces Hearing Aids; Laborie Medical to Acquire Urotronic; Synchron’s Brain-Computer Interface Trial
Quest Diagnostics Granted FDA Breakthrough Designation for its Adeno-associated virus (AAV) Test On August 30, 2023, Quest Diagnostics announced that its AAVrh74 ELISA assay (CDx) has been granted Breakthrough Device Designation from the US Food and Drug Administration (FDA). The enzyme-linked immunosor...
Read More...
Aug 30, 2023
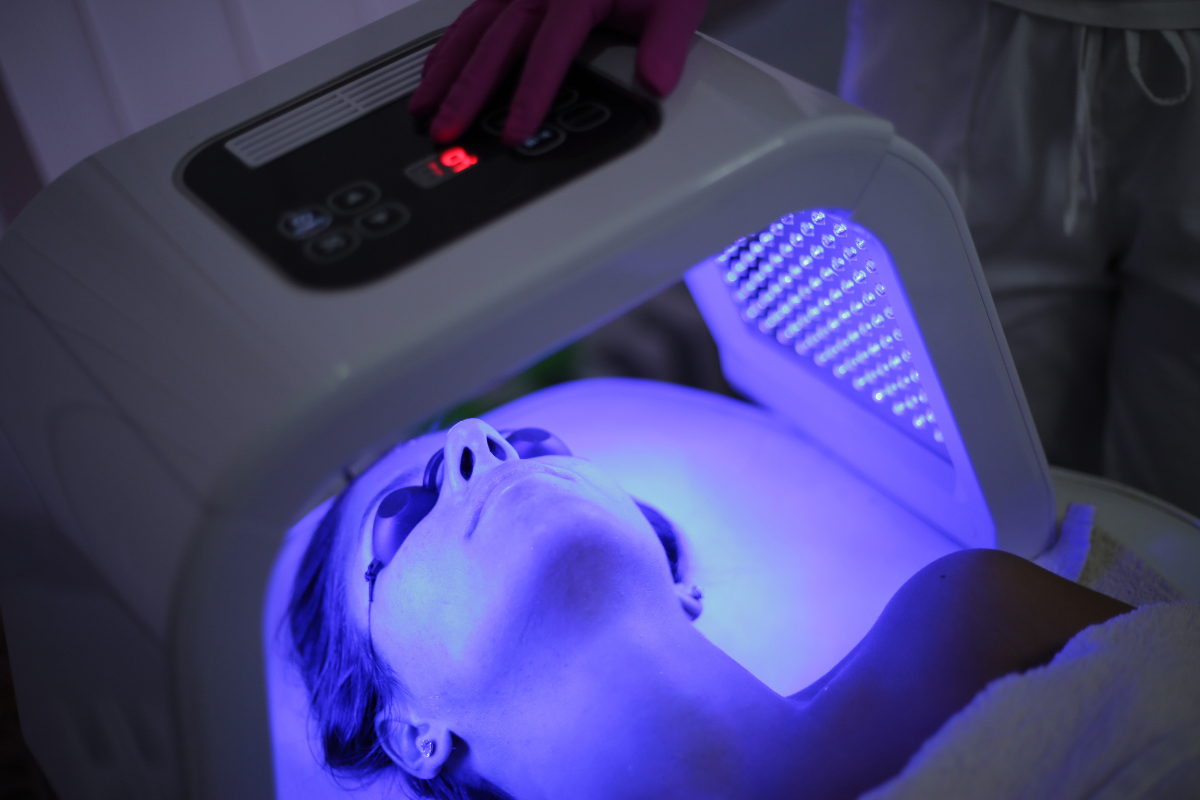
Harnessing Light for Health: Evaluating the Growing Demand for Light Therapy
In recent years, the rapid development of technology and innovation has sped up the transformation of the healthcare and well-being industry. Among the various emerging and evolving trends, Light Therapy is one of the key technologies gaining immense attention among the users. Light Therapy is an ancient practice, ...
Read More...
Aug 24, 2023
Smith+Nephew’s Hip Arthroplasty System; Mammotome Launched the HydroMARK Plus Breast Biopsy Site Marker; FDA Clearance for Pioneering MARS System; CE Marks for NGS-based Test Kits; BaroPace Updated on Non-Pharmacologic Hypertension and Heart Failure Treatment Trial; Quanta Completed Enrollment in Home Run Study
Smith+Nephew Launched Hip Arthroplasty System in India On August 17, 2023, The London-based orthopedic device maker Smith+Nephew announced the launch of its OR3O dual mobility system for use in primary and revision hip arthroplasty in India. The dual mobility implants have a smaller-diameter femoral hea...
Read More...
Aug 17, 2023
4WEB Medical’s Cervical Spine Plating Solution; Viseon Launched Visualization System for Minimally Invasive Spine Surgery; ZimVie’s New Mobi-C Implant; GE HealthCare’s Wireless Monitoring Solution; Acorai’s Non-Invasive Intracardiac Pressure Monitor; CytoSorbents’s STAR-T Pivotal Trial
4WEB Medical Launched its Cervical Spine Plating Solution On August 15, 2023, 4WEB Medical, an orthopedic implant company focused on developing innovative implants that utilize its proprietary Truss Implant Technology™, launched the newest addition to the company's implant portfolio, the Cervical Spine Pla...
Read More...
Aug 10, 2023
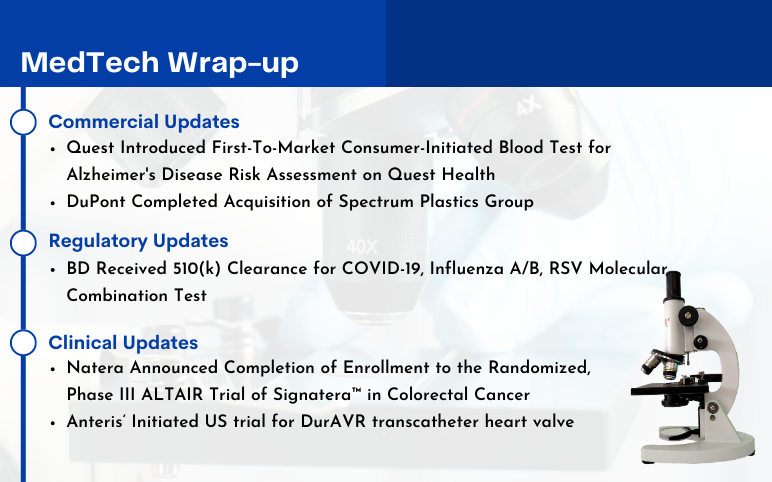
Quest Introduced Consumer-Initiated Blood Test for Alzheimer’s Disease Risk Assessment; DuPont Acquires Spectrum Plastics; BD Received 510(k) Clearance for Molecular Combination Test; Natera Updates on Phase III ALTAIR Trial of Signatera; Anteris’ DurAVR transcatheter heart valve Trial
Quest Introduced First-To-Market Consumer-Initiated Blood Test for Alzheimer's Disease Risk Assessment on Questhealth Website On July 31, 2023, Quest Diagnostics, a leader in diagnostic information services, announced the introduction of the AD-Detect™ Test for Alzheimer's Disease on questhealth.com – the first ...
Read More...
Aug 09, 2023
Diagnostic Precision: The Rise of Medical Imaging Technologies and Market Trends
In recent years, significant strides have been made in the realm of medical imaging, ushering in a new era of possibilities for healthcare professionals engaged in diagnosing, treating, and monitoring a diverse array of medical conditions and diseases. The convergence and developments in the state-of-the-art techno...
Read More...
Aug 03, 2023
Icotec’s VADER Pedicle System; Terumo BCT’ Reveos Automated Whole Blood Processing System; Genesis MedTech’s J-Valve Transfemoral System; STARmed Launched STARmed America in the USA
Genesis MedTech Announces FDA Breakthrough Device Designation for the J-Valve™ Transfemoral System Genesis MedTech, a leading medical device company, announced that the US Food and Drug Administration (FDA) has designated its J-ValveTM Transfemoral (TF) System as a Breakthrough Device. This innovative heart valv...
Read More...
Jul 27, 2023
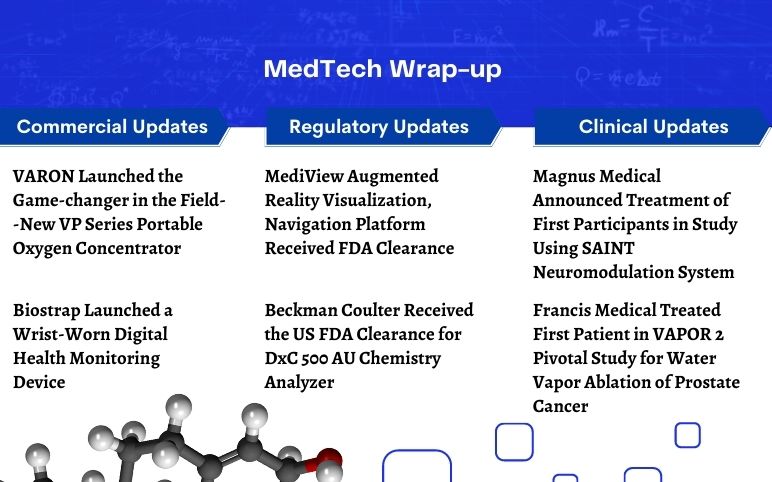
VARON’s New VP Series Portable Oxygen Concentrator; Biostrap’s Wrist-Worn Digital Health Monitoring Device; MediView’s AR Navigation Platform; Beckman Coulter’s DxC 500 AU Chemistry Analyzer; Magnus Updated on Study Using SAINT Neuromodulation System; Francis Medical’s VAPOR 2 Pivotal Study
VARON Launched the Game-changer in the Field--New VP Series Portable Oxygen Concentrator On July 19, 2023, VARON, a leading oxygen concentrator manufacturer, announced the launch of a game-changer portable oxygen concentrator VP-2. A new member of the VP series, VP-2 is incorporated with innovative tech...
Read More...
Jul 26, 2023
Innovative Solutions, Lifesaving Possibilities: Evaluating the Artificial Organs Market Dynamics
Over the past few years, there has been a remarkable advancement in the artificial organs market. Cutting-edge research, technological innovations, and breakthroughs in the artificial organ segment have revolutionized healthcare and extended the lives of countless individuals facing organ failure or similar impairm...
Read More...
Jul 13, 2023
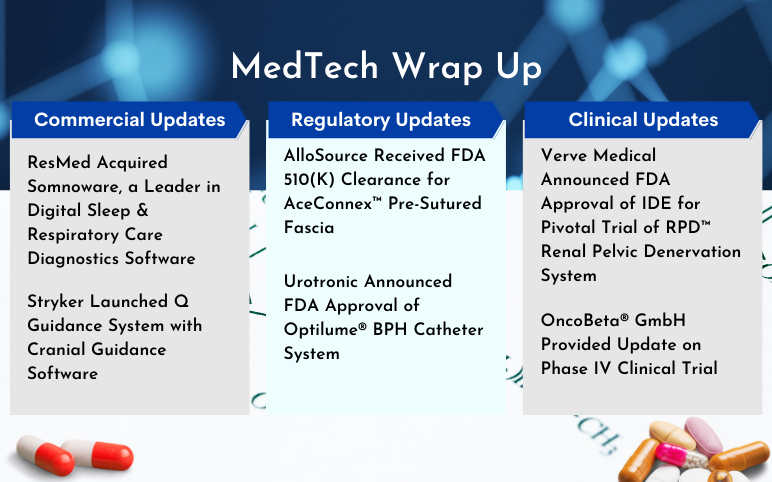
ResMed Acquired Somnoware; Stryker Launched Q Guidance System; AlloSource’s AceConnex Pre-Sutured Fascia; Urotronic’s Optilume BPH Catheter System; Verve Medical’s Pivotal Trial of RPD Renal Pelvic Denervation System; OncoBeta Provided Update on Rhenium-SCT
ResMed Acquired Somnoware, a Leader in Digital Sleep and Respiratory Care Diagnostics Software On July 05, 2023, ResMed acquired privately held Somnoware, a US leader in sleep and respiratory care diagnostics software. The Somnoware software streamlines the processes of physicians as well as sleep and p...
Read More...




-Agonist.png)

