NASH
Sep 04, 2024
Empowering Change: REZDIFFRA’s Trailblazing Journey in NASH Treatment
Liver disease emerges as a burgeoning global health concern, presenting a myriad of formidable challenges. From viral hepatitis to alcoholic liver disease, nonalcoholic fatty liver disease (NAFLD), liver cirrhosis, and hepatocellular carcinoma (HCC), these conditions collectively afflict millions, exacting a toll o...
Read More...
Jun 11, 2024
Moderna’s Phase III Trials for Dual Influenza and COVID-19 Vaccine; Almirall’s Klisyri FDA Approval; Lilly’s Tirzepatide MASH Trial; Cycle Pharmaceuticals Acquisition of Vanda Pharmaceuticals; Ipsen’s Iqirvo FDA Approval
Moderna Reports Successful Phase III Trials for Dual Influenza and COVID-19 Vaccine Moderna, Inc. has reported that its Phase III trial for mRNA-1083, an experimental combination vaccine targeting both influenza and COVID-19, achieved its main objectives by generating a stronger immune response than the approved...
Read More...
Mar 19, 2024
Bristol Myers Squibb’ Karuna Therapeutics Buyout; Orchard Therapeutics’ Lenmeldy FDA Approval; Madrigal Pharmaceuticals’ Rezdiffra FDA Approval; AstraZeneca’s Acquisition of Amolyt Pharma; Prilenia’s Pridopidine EU Marketing Approval
Bristol Myers Squibb Enhances Neuroscience Arm with Karuna Therapeutics Buyout Bristol Myers Squibb has declared the finalization of its purchase of Karuna Therapeutics, Inc. With this acquisition concluded Karuna’s shares are no longer being traded on the Nasdaq Global Select Market, as Karuna is now fully owne...
Read More...
Jul 07, 2023
A Beacon of Hope: A Look at the Latest Advances in Emerging Therapies for Liver Cirrhosis Treatment
Liver cirrhosis is a chronic and progressive condition characterized by the gradual replacement of healthy liver tissue with scar tissue, known as fibrosis. This scarring disrupts the normal structure and function of the liver. It also leads to symptoms such as confusion, ascites, pruritus, weakness, fatigue, and b...
Read More...
Oct 04, 2022
Zealand Pharma’s Phase III Results of Glepaglutide; FDA Approves Amylyx’s ALS Drug Relyvrio; Novo Nordisk and Ventus Therapeutics Signs Licencing Deal; FDA Approves Futibatinib; Sarepta Files Duchenne Muscular Dystrophy for FDA Approval; Biogen and Eisai’s Lcanemab Phase III Study
Zealand Pharma Announces the Positive Topline Results from its Phase III trial of Glepaglutide Zealand Pharma A/S, a biotech company specializing in peptide-based medicines, announced positive topline results from its phase III trial of glepaglutide. In the evenly randomized double-blind trial, 106 patients with...
Read More...
Sep 20, 2022
AstraZeneca’s Danicopan Trial; CHMP Recommends Sanofi/AstraZeneca’s nirsevimab; Akero’s NASH Drug Trial; FDA Grants Orphan Drug Status to SY-5609; BMS’s Opdivo Trial Results; Pfizer to File for FDA Approval for Meningitis Vaccine; EMA Orphan Drug Designation to CAN-2409; FDA Starts Priority Review of Chiesi ‘s velmanase alfa
AstraZeneca’s Danicopan Shows Positive Results in Phase III Trial Danicopan, an oral Factor D inhibitor developed by AstraZeneca, was expected to fail a phase II trial in rare kidney disease in 2020, but a new readout could revive the drug. Danicopan (ALXN2040) has demonstrated efficacy as an adjunct treatment f...
Read More...
Jun 29, 2021
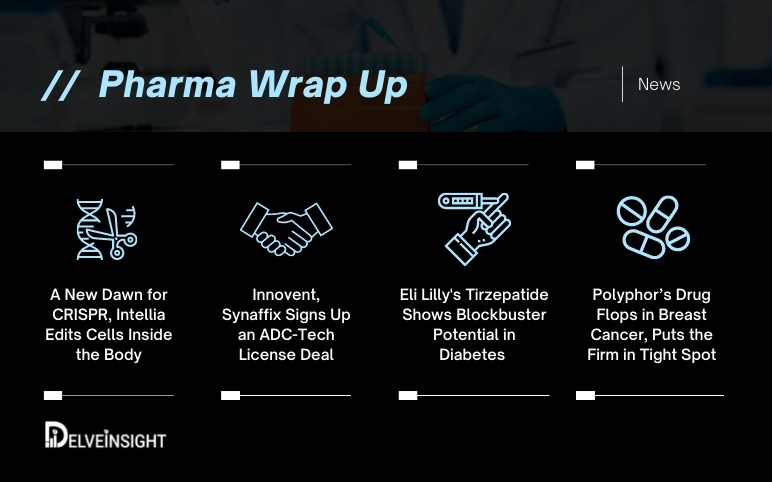
Intellia’s Quest with CRISPR; Innovent, Synaffix ADC Tech Deal; Lilly’s Diabetes Blockbuster Tirzepatide; Polyphor’s Bleak Future
A New Dawn for CRISPR, Intellia Edits Cells Inside the Body Intellia Therapeutics, along with its partner Regeneron Pharmaceuticals, recently announced its successful attempt in editing the cells inside the body leveraging the CRISPR technique. The world’s first-ever trial that has their DNA edited through an in...
Read More...
May 25, 2021
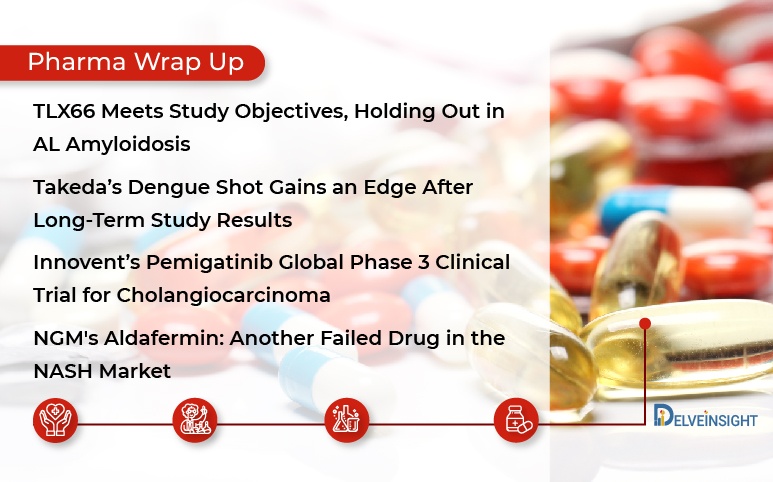
TLX66 in AL Amyloidosis; Takeda’s Dengue Shot; Innovent’s Cholangiocarcinoma Clinical Trial; Another Failed NASH Drug
TLX66 Meets Study Objectives, Holding Out in AL Amyloidosis Telix Pharmaceuticals reported the results from the TRALA trial (Targeted Radiotherapy for AL Amyloidosis), a Phase I/IIa trial evaluating the safety and toxicity of TLX66 as the sole bone marrow conditioning agent prior to autologous hematopoietic stem...
Read More...
Dec 15, 2020
FTA for Gannex’s ASC4; Disappointment for Incyte’s Ruxolitinib; Historic win for Pfizer, BioNTech’s COVID Vaccine; Alexion buyout; the debut of InnoSkel
Gannex received US FDA fast track designation for its NASH drug, ASC42 an FXR Agonist Gannex Pharma has received Fast Track designation approval from FDA for its drug candidate ASC42 for non-alcoholic steatohepatitis (NASH). The FTA designation will help the pharma company to advance its research and development...
Read More...
Jul 16, 2020
Orchard licenses gene therapy tech from GSK; Glympse raises $46M; FDA voted to recommend Terlipressin; Breast Cancer research update
Orchard licenses the gene therapy technology from GlaxoSmithKline Orchard Therapeutics has permitted lentiviral stable cell line technology from GlaxoSmithKline for its use in hematopoietic stem cell gene therapies. GSK filed for patents on the tech before selling its rare disease gene therapy portfolio to...
Read More...






-Agonist.png)

