A Beacon of Hope: A Look at the Latest Advances in Emerging Therapies for Liver Cirrhosis Treatment
Jul 07, 2023
Table of Contents
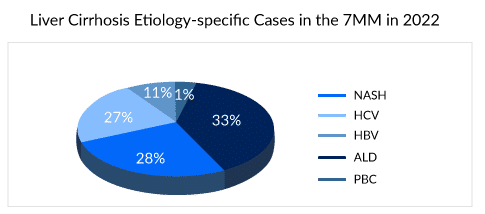
Liver cirrhosis is a chronic and progressive condition characterized by the gradual replacement of healthy liver tissue with scar tissue, known as fibrosis. This scarring disrupts the normal structure and function of the liver. It also leads to symptoms such as confusion, ascites, pruritus, weakness, fatigue, and bloating. As per DelveInsight’s Liver Cirrhosis Epidemiology Forecast report, liver cirrhosis affects more than 3 million people in the 7MM, of which the US alone accounts for about 60% of cases. The disease can be caused by various factors, including chronic alcohol abuse, viral hepatitis, non-alcoholic steatohepatitis (NASH), and autoimmune diseases where ALD, HCV, and NASH comprise of nearly 85% of the cases.
What are the current liver cirrhosis management approaches?
The management of liver cirrhosis focuses on a multidisciplinary approach that involves hepatologists, gastroenterologists, and other healthcare professionals. The primary goals of liver cirrhosis treatment include halting disease progression, preventing complications, and improving quality of life. Liver cirrhosis treatment strategies involve lifestyle changes, such as abstaining from alcohol, maintaining a healthy diet, and regular exercise to manage the symptoms and prevent the progression of liver damage.
Downloads
Click Here To Get the Article in PDF
Recent Articles
No approved therapies have been available to treat NASH, fibrosis, or liver cirrhosis. However, in recent years, there have been multiple companies and academic centers that are examining various agents for liver cirrhosis treatment. Significant advancements have been made in developing emerging liver cirrhosis therapies bringing new hope to patients with this debilitating condition.
What are the current unmet needs in liver cirrhosis treatment?
While significant advancements have been made in treating liver cirrhosis, there is still a lack of effective therapies for the advanced stages of the disease. Once cirrhosis has progressed to a severe stage, the possibility of slowing or reversing the fibrotic process becomes limited. Liver fibrosis is a key feature of cirrhosis and is associated with poor prognosis. Therapies that can effectively reverse fibrosis and prevent the progression of cirrhosis are a high unmet medical need. Different causes of cirrhosis have different underlying mechanisms; targeted therapies are needed to address these specific causes. Moreover, personalized treatment approaches need to be considered to treat patients with significant variations in the underlying mechanisms, disease progression, and response to treatment.
Potential emerging drugs to revolutionize the current liver cirrhosis treatment market
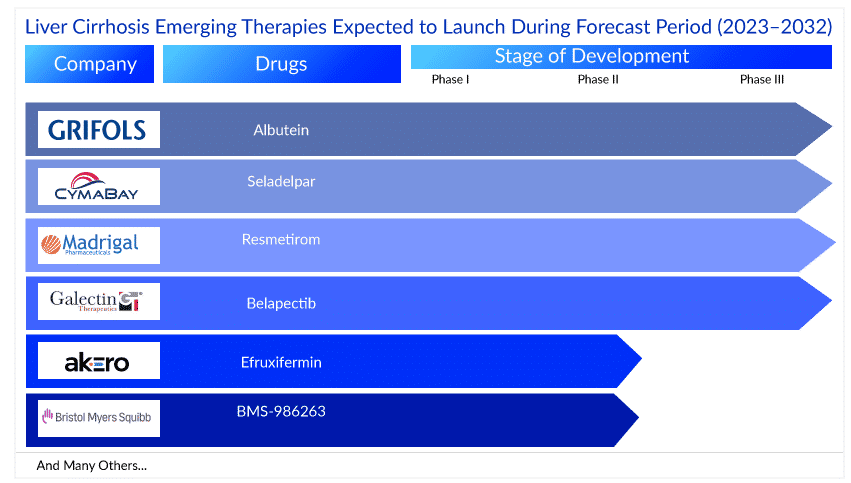
The liver cirrhosis treatment market is witnessing promising advancements, with several emerging drugs that have shown potential to revolutionize the current market. Prominent pharmaceutical players such as Madrigal Pharmaceuticals, Inc., Galectin Therapeutics Inc., CymaBay Therapeutics, Inc., Akero Therapeutics, Inc, NGM Biopharmaceuticals, Inc, Novo Nordisk A/S, Bristol-Myers Squibb, and others are already treading into the liver cirrhosis treatment market and expect to be well received.
Madrigal’ MGL-3196 (Resmetirom), Akero’s Efruxifermin (EFX), Bristol’s BMS-986263, and Galectin’s Belapectin are a few therapies focused on the NASH cirrhosis population while CymaBay’s Seladelpar is being developed for PBC cirrhosis patients and ALBUTEIN 20% injectable solution for decompensated cirrhosis associated with HBV and HVC patients.
Madrigal’s Resmetirom, also known as MGL-3196, is a novel selective thyroid hormone receptor beta-agonist being investigated as a potential treatment for NASH and liver cirrhosis in a Phase III clinical trial. In a Phase II clinical trial involving patients with NASH and liver fibrosis, resmetirom demonstrated a significant reduction in liver fat content, improvement in liver enzymes, and reduction in markers of fibrosis. These findings suggest its potential as a treatment for liver cirrhosis; thus, the company initiated a Phase III trial for the F4 NASH population.
Another investigational drug, Akero’s Efruxifermin (also known as AKR-001), is a long-acting fibroblast growth factor 21 (FGF21) analog being evaluated in a Phase IIb/III trial for NASH-associated cirrhosis. Efruxifermin is considered an exciting and potentially ground-breaking emerging treatment as the drug has already proved, in mid-stage trials, the clinical efficacy required to improve fibrosing and resolve NASH. As research progresses and clinical trials unveil more substantial evidence, the prospect of Efruxifermin as the best-emerging treatment for liver cirrhosis patients becomes increasingly attractive, igniting optimism in the market.
Both Phase III drugs, Efruxifermin and Resmetirom, are expected to gain a leading edge in the market when approved. According to DelveInsight’s Liver Cirrhosis Market Forecast report, Efruxifermin and Resmetirom are anticipated to launch around the same time and become blockbuster therapies by 2032, having the potential to garner peak sales of nearly USD 1.5 billion and USD 1.1 billion, respectively by 2032 in the 7MM.
BMS-986263, a small interfering RNA being evaluated in a Phase II trial for NASH cirrhosis, has also garnered considerable attention in the field of liver cirrhosis. However, according to DelveInsight’s Liver Cirrhosis Market report, while BMS-986263 holds immense promise as a treatment for liver cirrhosis, it is anticipated to arrive later in the liver cirrhosis space compared to Efruxifermin and Resmetirom. By the time BMS-986263 enters the market, Efruxifermin and Resmetirom would have captured considerable significance and market share. Although this may create a sense of anticipation among researchers, it also provides an opportunity to learn from the progress and challenges faced by the earlier candidates. The delayed arrival of BMS-986263 allows BMS to thoroughly understand the treatment landscape, fostering a comprehensive approach to developing this potentially ground-breaking therapy. As such, while Efruxifermin and Resmetirom take center stage in the liver cirrhosis space, the arrival of BMS-986263 is eagerly awaited, offering the potential for further advancements and contributing to the growing arsenal of innovative treatments for liver cirrhosis.
Galectin’s Belapectin (GR-MD-02), a galectin 3 inhibitor, is being studied to treat NASH cirrhosis in Phase II/III to evaluate its safety and effectiveness in a larger patient population. In a clinical trial, belapectin showed potential in improving liver markers in a few cirrhosis patients. It is worth noting that Galectin has a long way to go to demonstrate Belapectin’s efficacy in clinical trials. According to DelveInsight’s analysis, while with current results available, it is predicted that it may not capture the same level of attention as some of its counterparts, Belapectin will bring its unique potential to the table. The drug has also received fast-track designation from the US Food and Drug Administration (FDA) for treating NASH cirrhosis. This biweekly IV injection is in Phase II/III and is expected to enter the US liver cirrhosis market by 2026.
CymaBay Therapeutics, Inc. is developing Seladelpar, a peroxisome proliferator-activated receptor delta (PPARδ) agonist, in Phase III clinical trial for PBC cirrhosis. Although the drug demonstrated potential in targeting the underlying mechanisms of liver cirrhosis, concerns regarding liver toxicity observed in some patients during clinical trials have caused distress. This may create an opportunity for other emerging therapies, such as Efruxifermin and Resmetirom, to gain momentum and capture a larger market share. However, the potential of Seladelpar should not be overlooked, as ongoing research and further evaluation may help shed light on its efficacy and safety profile. Apart from the above therapies, ALBUTEIN 20% injectable solution (human albumin) is a protein-based therapy commonly used to treat liver cirrhosis and is currently being investigated for decompensated cirrhosis due to HBV and HCV. The drug is anticipated to garner a relatively small market size compared to other emerging treatments in the liver cirrhosis space. This is primarily due to the smaller patient population affected by this specific subtype of cirrhosis. As ALBUTEIN focuses on addressing the needs of a niche group of individuals, the potential market size naturally becomes limited. One potential advantage of human albumin is that it is a well-established therapy with a long history of use in clinical practice. Though the market size may be smaller, the impact of ALBUTEIN is significant for those who will benefit from its unique approach, highlighting the importance of diverse therapies in the liver cirrhosis treatment space.
With the anticipated launch of these emerging therapies, the liver cirrhosis treatment market is poised to undergo a significant transformation. These ground-breaking treatments offer new hope and possibilities for patients grappling with this complex condition. This shift will not only benefit patients but also stimulate competition, foster advancements in research and development, and drive innovation within the field. As per DelveInsight’s analysis, the liver cirrhosis market is anticipated to grow and reach a market size of USD 6.7 billion in the 7MM by 2032.
How can emerging therapies for liver cirrhosis treatment address unmet needs in treating this condition?
The current liver cirrhosis treatment has significant toxicity and side effects. They are limited to managing the complications, not the cure. Emerging therapies appear to have the potential to address these unmet medical needs of patients by providing improved efficacy, reduced toxicity, disease modification, and improving the overall treatment experience for patients. Some emerging therapies for liver cirrhosis are being developed as combination therapies, for example, Semaglutide (SEMA) in combination with Cilofexor (CILO) and Firsocostat (FIR), that target multiple mechanisms underlying the disease. This approach can address the unmet need for more comprehensive and effective liver cirrhosis treatments to address the multiple aspects of liver cirrhosis.
Despite promising developments, challenges lie ahead, including ensuring the safety and efficacy of these drugs, navigating regulatory hurdles, and addressing the high cost of these therapies. The potential benefits of these emerging therapies for liver cirrhosis treatment, including improved outcomes and quality of life for patients, make it an exciting time in research and treatment. As research around emerging therapies for liver cirrhosis advances rapidly, it provides a beacon of hope for patients and physicians alike.
FAQs
Liver Cirrhosis is a late-stage liver disease in which healthy liver tissue is replaced with scar tissue and the liver is permanently damaged.
Cirrhosis is most commonly caused by hepatitis C virus (HCV), alcoholic liver disease, and nonalcoholic steatohepatitis (NASH) in the developed world, whereas hepatitis B virus (HBV) and HCV are the most common causes in the poor world. Cirrhosis can also be caused by other conditions such as autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, hemochromatosis, Wilson disease, alpha-1 antitrypsin deficiency, Budd-Chiari syndrome, drug-induced liver cirrhosis, and chronic right-sided heart failure.
Nodules and abnormalities form both inside and outside the liver, and these changes can be discovered via an ultrasonic examination or a CT scan. Because liver function steadily declines, liver cirrhosis frequently goes unreported and unacknowledged. As a result, it is critical to have regular examinations.
Cirrhosis treatment techniques may differ depending on the disease stage and underlying etiology. The goals of treatment for compensated cirrhosis are to slow, stop, or reverse fibrosis progression and to prevent decompensation events, whereas the goals of treatment for decompensated cirrhosis are to prevent further decompensation and death and to treat complications related to portal hypertension.

Downloads
Article in PDF
Recent Articles
- AZ announces positive results for durvalumab and tremelimumab; FDA nods to extend LILETTA’s use t...
- Bristol Myers Squibb’ Karuna Therapeutics Buyout; Orchard Therapeutics’ Lenmeldy FDA Approval; Ma...
- Intellia’s Quest with CRISPR; Innovent, Synaffix ADC Tech Deal; Lilly’s Diabetes Bloc...
- EU approval for Libtayo and Dovato; Boehringer expands NASH pipeline
- Zealand Pharma’s Phase III Results of Glepaglutide; FDA Approves Amylyx’s ALS Drug Relyvrio; Novo...