obstructive sleep apnea
Jun 03, 2025
Beam’s BEAM-302 Earns FDA Orphan Drug Tag for AATD; Incannex’s IHL-42X Moves to Phase III After FDA Protocol OK; Kura and Kyowa Kirin’s Ziftomenib Gets FDA Priority Review in NPM1-Mutant AML; GSK’s Linerixibat NDA Accepted by FDA for PBC-Linked Pruritus; Ascendis’ TransCon CNP Granted FDA Priority Review for Achondroplasia
Beam Therapeutics Secures FDA Orphan Drug Designation for BEAM-302 in AATD Beam Therapeutics Inc. announced that the FDA has granted Orphan Drug Designation to its investigational therapy, BEAM-302, for the treatment of alpha-1 antitrypsin deficiency (AATD). BEAM-302 is a liver-targeting lipid nanoparticle (LNP)...
Read More...
Jan 06, 2025
Lilly’s ZEPBOUND Clears Hurdle in Sleep Apnea Treatment
Eli Lilly’s blockbuster weight loss GLP-1 drug ZEPBOUND has received FDA approval to treat obstructive sleep apnea in adults with obesity, alongside diet and exercise. This marks the first FDA-approved medication for obstructive sleep apnea. The approval adds a second indication for ZEPBOUND, following its...
Read More...
Dec 24, 2024
FDA Approves Vertex’s ALYFTREK for Cystic Fibrosis; ZEPBOUND Gets FDA Approval for Obstructive Sleep Apnea in Obese Adults; Tonix’s TNX-102 SL NDA Accepted for Fibromyalgia; Quoin’s QRX003 Clears FDA for Netherton Syndrome Study; FDA Approves First Mesenchymal Stromal Cell Therapy for Acute GvHD
Vertex Receives US FDA Approval for ALYFTREK: A Breakthrough CFTR Modulator for Cystic Fibrosis Vertex Pharmaceuticals Incorporated announced that the FDA had approved ALYFTREK (vanzacaftor/tezacaftor/deutivacaftor), a once-daily triple combination CFTR modulator for the treatment of cystic fibrosis. ALYFTREK is...
Read More...
Dec 26, 2023
ImPact Biotech’s IND Application for Padeliporfin VTP; Orphan Drug Designation to Ocelot Bio’s OCE-205 for Ascites; Amylyx’s Phase 3 ORION Study of AMX0035 for PSP; SELLAS Receives FDA Orphan Drug Designation for SLS009 for PTCL Treatment; Apnimed Updated on Second Phase 3 Clinical Study of AD109 for OSA
ImPact Biotech Receives FDA Clearance of IND Application for Padeliporfin VTP in Pancreatic Cancer ImPact Biotech, a biotechnology company in its clinical stage dedicated to advancing Padeliporfin Vascular Targeted Photodynamic (VTP) therapy for various solid tumors, announced on December 20, 2023, that the U.S....
Read More...
Sep 20, 2023
Sleep Aids Market: Examining Cutting-Edge Technologies and Major Breakthroughs
Sleep Aids Market: Examining Cutting-Edge Technologies and Scientific Breakthroughs Sleep can be burdensome when it disrupts daily routines, requires a significant time commitment, and is marred by sleep disorders or disturbances that affect overall well-being. In today's fast-paced world, where stress, anxiety,...
Read More...
Aug 16, 2023
Exploring the World of Anti-snoring Devices: A Comprehensive Market Assessment
Snoring, a common sleep-related issue, can disrupt both the snorer's sleep and that of their partner or roommate. As per the estimate, about 90 million Americans suffer from snoring, either temporarily or semi-permanently, due to colds, allergies, or other factors. Snoring can have several implications, and it can ...
Read More...
May 30, 2023
ATS 2023 Updates: AD109’s Potential as the First Oral Medication for Obstructive Sleep Apnea (OSA)
Apnimed’s AD109 has the potential to be the first oral pharmaceutical drug that remedies both, the primary cause of OSA, nocturnal airway obstruction, and the daytime symptoms of OSA, such as fatigue. The dual mode of action of AD109 leads to upper airway dilatation as well as enhanced breathing and oxygen...
Read More...
Jan 12, 2023
Vivos Therapeutics ‘s DNA Oral Appliance for the Treatment of OSA; Arthrex’s TightRope Implant for Pediatric ACL Surgery; Chindex Medical Acquired 10 ViewRay MRIdian Systems; Zimmer Biomet to Acquire Embody; GE HealthCare to buy IMACTIS; Glaukos Announced the iDose TR Exchange Trial
Vivos Therapeutics Received FDA 510(k) Clearance for its Flagship DNA Oral Appliance for the Treatment of Obstructive Sleep Apnea On January 4, 2023, Vivos Therapeutics, Inc., a medical technology company focused on developing innovative treatments for patients suffering from dentofacial abnormalities and/or mil...
Read More...
May 30, 2022
Covering the Therapeutic Advancements in Obstructive Sleep Apnea Treatment Scenario
Obstructive Sleep Apnea (OSA) is a common, chronic, sleep-related breathing disorder characterized by periodic narrowing and obstruction of the pharyngeal airway during sleep, leading to either complete or partial obstruction of the airway, resulting in apneas, hypopneas, or both. Obstructive Sleep Apnea causes day...
Read More...
Mar 03, 2022
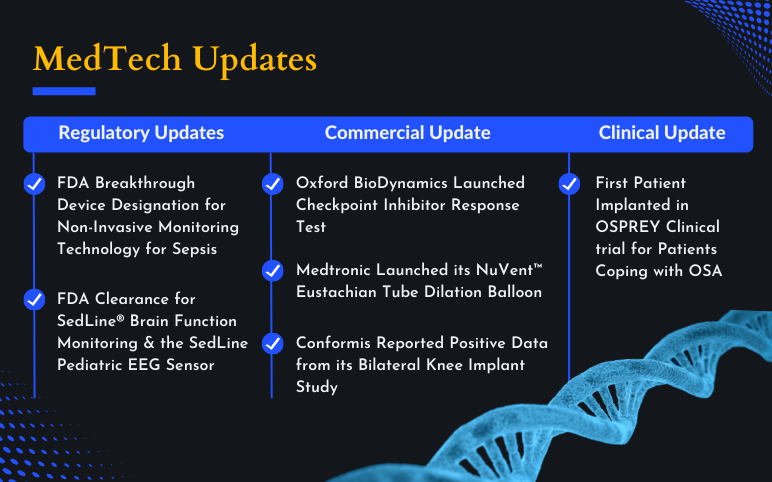
Noninvasix’s LIVOx™ Central Venous Oxygenation Monitor; LivaNova’s aura6000 System; Oxford BioDynamics’s EpiSwitch® CiRT; Masimo’s SedLine® Brain Function Monitoring and the SedLine Pediatric EEG Sensor; Medtronic’s NuVent™ Eustachian Tube Dilation Balloon; Conformis’s published data for Bilateral Knee Implant Study
Noninvasix Granted the US FDA Breakthrough Device Designation for Non-Invasive Monitoring Technology for Sepsis On February 23, 2022, Noninvasix, Inc. received the US FDA breakthrough device designation for its LIVOx™ Central Venous Oxygenation Monitor. It is a non-invasive device and provides real-time, ...
Read More...








-Agonist.png)

