ProMIS Neurosciences
May 09, 2023
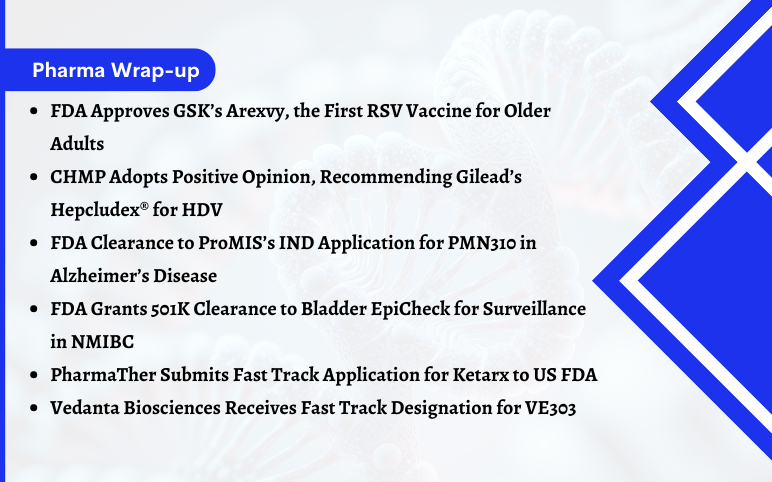
FDA Approves GSK’s Arexvy for RSV; CHMP’s Opinion on Gilead’s Hepcludex® for HDV; FDA Clearance to ProMIS’s IND Application for PMN310; FDA Grants 501K Clearance to Bladder EpiCheck; PharmaTher Submits Fast Track Application for Ketarx to US FDA; Fast Track Designation to Vedanta Biosciences’ VE303
FDA Approves GSK’s Arexvy, the First RSV Vaccine for Older Adults GSK plc stated that the US Food and Drug Administration (FDA) has approved Arexvy (respiratory syncytial virus vaccine, adjuvanted) for the prevention of lower respiratory tract disease (LRTD) caused by a respiratory syncytial virus (RSV) in peopl...
Read More...
-Agonist.png)

