Radiofrequency Ablation Devices
Jan 28, 2026
Advances in Varicose Vein Treatment Devices: Technologies Shaping Modern Venous Care
Varicose veins, enlarged, tortuous veins most commonly occurring in the lower extremities, impact millions worldwide, causing discomfort, cosmetic concerns, and, in severe cases, complications such as venous insufficiency or ulcers. While traditional varicose vein treatments such as compression stockings and surgic...
Read More...
Jul 17, 2025
Medtronic Gains CE Mark for LigaSure™ on Hugo™ Robotic System; J&J Wins FDA Nod for VARIPULSE™ Irrigation Update; DSMB Clears Continuation of Aethlon Medical Clinical Study; MicroPort® CardioFlow Marks First VitaFlow Liberty® Implants in South Korea; Olympus Unveils EU-ME3 Ultrasound Processor in U.S.; QuidelOrtho, BÜHLMANN Launch fCAL® & fPELA® Turbo Assays on VITROS™
Medtronic Secured CE Mark for LigaSure™ Technology Integration on Hugo™ Robotic-Assisted Surgery System, Paving the Way for the Future of Surgery On July 15, 2025, Medtronic plc, a global leader in healthcare technology, announced that it received CE Mark approval for its LigaSure™ RAS vessel-sealing techn...
Read More...
May 22, 2025
FDA Clears Fujirebio’s Lumipulse® G Plasma Test for Detecting Alzheimer’s-Linked Amyloid Pathology; Stryker Gains FDA Clearance for OptaBlate® BVN System to Treat Chronic Low Back Pain; Abbott Unveils Clinical Evidence Linking Libre® CGM to Reduced Cardiovascular Events in Diabetes; GE HealthCare Launches AI-Based CleaRecon DL for Enhanced 3D Reconstruction in Interventional Suites; Philips Advances Cardiac Care with Launch of RADIQAL Clinical Study and Real-Time 3D Intracardiac Imaging Catheter in Europe
Fujirebio Received FDA Clearance for Innovative Lumipulse® G Plasma Biomarker Test, Marking Major Advancement in Identifying Amyloid Pathology Linked to Alzheimer’s Disease On May 16, 2025, Fujirebio announced that the U.S. Food and Drug Administration (FDA) granted 510(k) clearance for its Lumipulse® G pT...
Read More...
Dec 12, 2024
Stereotaxis and Shanghai MicroPort EP Medtech Co., Ltd. Receives Approval for Magbot Robotic Magnetic Navigation Ablation Catheter From China’s NMPA; AngioDynamics NanoKnife® System Approved by FDA for Prostate Tissue Treatment; Zynex Successfully Completes Laser Pulse Oximetry Clinical Trial; COTA, PreciseDx, and Baptist Health Validate AI-Powered PreciseBreast as Equivalent to Oncotype DX; Medtronic Introduces Percept™ RC Rechargeable Neurostimulator; Crown Aesthetics Raises the Bar with the Introduction of SkinPen® Precision Elite
China's NMPA Approved Magbot Robotic Magnetic Navigation Ablation Catheter On December 9, 2024, Stereotaxis and Shanghai MicroPort EP Medtech Co., Ltd., announced that the Magbot™ Magnetic Navigation Ablation Catheter received regulatory approval from China’s National Medical Products Administration (NMPA). T...
Read More...
Sep 12, 2024
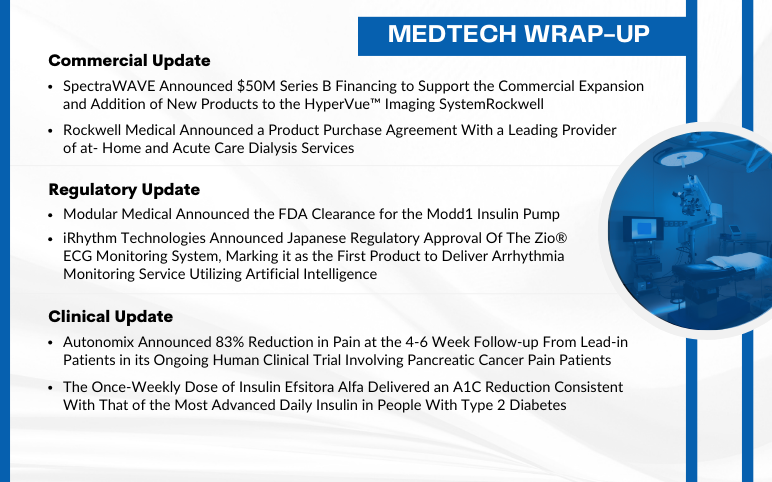
SpectraWAVE raised $50M; Rockwell Medical signed dialysis deal; Modular Medical got FDA nod for Modd1 pump; iRhythm’s Zio approved in Japan; Autonomix trial showed 83% pain reduction; Insulin Efsitora Alfa improved A1C.
Modular Medical Announced the FDA Clearance for the Modd1 Insulin Pump On September 5, 2024, Modular Medical, Inc., an insulin delivery system technology company, announced that it received the U.S. Food and Drug Administration (FDA) clearance to market and sell its MODD1 pump in the United States. With it...
Read More...
Dec 08, 2022
Olympus Launches New Gynecologic Power Morcellator; Journey Biosciences Launched NaviDKD; GE Board Approves Separation of GE HealthCare; FDA Approval to Enovis’s STAR Ankle; FDA Clearance to Deep Blue Medical’s T-Line Hernia Mesh; NeuroOne Completed Feasibility Study with its OneRF Ablation System
Olympus Launches New Gynecologic Power Morcellator for Its Contained Tissue Extraction System On November 30, 2022, Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, launched the moresolution™ Power Morcellator, manufactured by TROKAM...
Read More...




-Agonist.png)

