Respiratory Syncytial Virus Market
Jun 10, 2025
Amylyx’s AMX0114 Fast Tracked by FDA for ALS; Cellectar’s Iopofosine I 131 Granted FDA Breakthrough in Waldenstrom Macroglobulinemia; YolTech’s YOLT-101 Clears FDA IND for Familial Hypercholesterolemia; Oncovita’s MVdeltaC Gets FDA Orphan Tag for Pleural Mesothelioma; Merck’s ENFLONSIA Approved for RSV Prevention in Infants
Amylyx Pharmaceuticals Receives FDA Fast Track Designation for AMX0114 in ALS Amylyx Pharmaceuticals announced that the FDA has granted Fast Track designation to its investigational antisense oligonucleotide (ASO), AMX0114, for the treatment of amyotrophic lateral sclerosis (ALS). The candidate targets calpain-2...
Read More...
Jun 04, 2024
Moderna’s mRESVIA(R) FDA Approval; Novartis Scemblix® Phase III Data; Kite’s Tecartus for Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia; Astellas’ Zolbetuximab Biologics License Application; CHMP Recommends AstraZeneca’s Tagrisso
FDA Greenlights Moderna's RSV Vaccine mRESVIA(R) Moderna, Inc. has announced that the FDA has approved mRESVIA (mRNA-1345), an mRNA-based vaccine for respiratory syncytial virus (RSV), to protect adults aged 60 and over from lower respiratory tract infections caused by RSV. This approval, granted under a breakth...
Read More...
May 09, 2023
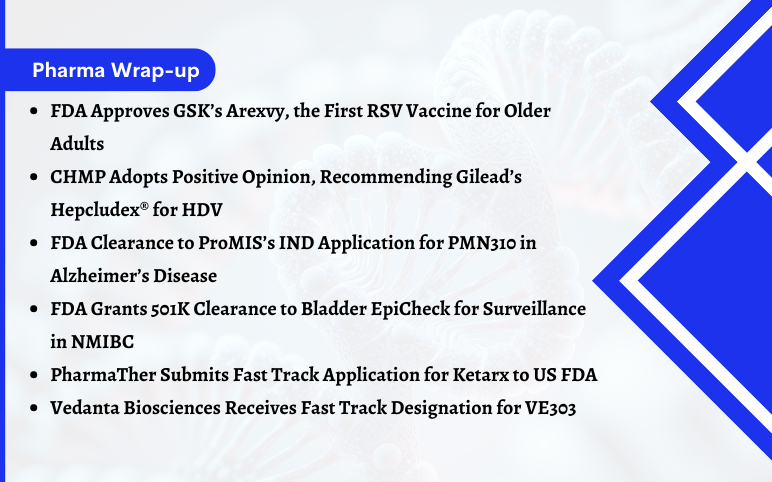
FDA Approves GSK’s Arexvy for RSV; CHMP’s Opinion on Gilead’s Hepcludex® for HDV; FDA Clearance to ProMIS’s IND Application for PMN310; FDA Grants 501K Clearance to Bladder EpiCheck; PharmaTher Submits Fast Track Application for Ketarx to US FDA; Fast Track Designation to Vedanta Biosciences’ VE303
FDA Approves GSK’s Arexvy, the First RSV Vaccine for Older Adults GSK plc stated that the US Food and Drug Administration (FDA) has approved Arexvy (respiratory syncytial virus vaccine, adjuvanted) for the prevention of lower respiratory tract disease (LRTD) caused by a respiratory syncytial virus (RSV) in peopl...
Read More...


-Agonist.png)

