Telehealth Market
Oct 01, 2025
Wearables 2.0: The Next Wave of Clinical-Grade Wearable Medical Devices
Not long ago, the word wearable instantly brought to mind fitness trackers counting steps or smartwatches nudging users to stand up. While those devices sparked the early boom, the next chapter, Wearables 2.0, is unfolding at the intersection of healthcare and technology. Today, wearable medical devices are no long...
Read More...
Mar 13, 2024
Telemedicine Service: Delving into Opportunities and Key Growth Factors Driving Market
Telemedicine Services have emerged as a transformative force in healthcare delivery, offering remote diagnosis, consultation, and treatment through telecommunications technology. This innovative approach to healthcare has garnered significant attention due to its potential to address various challenges plaguing tra...
Read More...
Oct 19, 2022
Evaluating the Key Trends and Technologies Shaping the Future of Dentistry
Oral diseases, while largely preventable, are one of the major health burdens globally and affect about 3.5 billion people. Dental caries or cavities (commonly known as tooth decay) and gum (periodontal) disease are the most common conditions that severely affect oral health and account for the most considerable pa...
Read More...
Sep 29, 2022
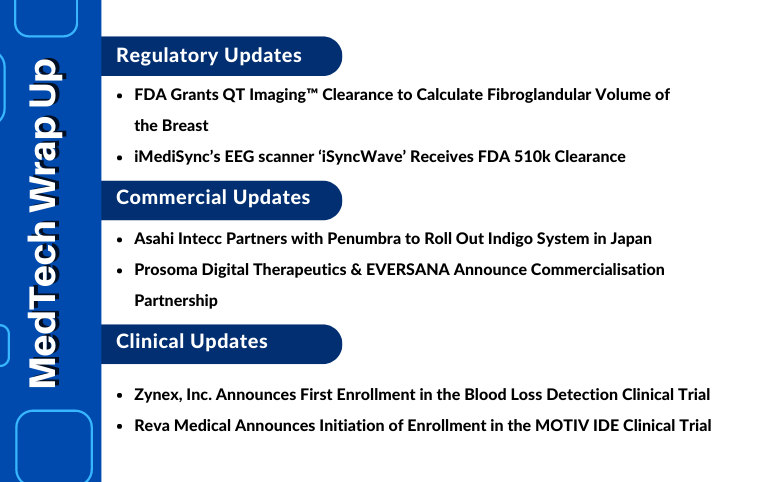
Asahi Intecc Partners with Penumbra; Prosoma and EVERSANA Announce Commercialisation Partnership; Zynex Starts Enrollment in the Blood Loss Detection Clinical Trial; Reva Initiates Enrollment in the MOTIV IDE Clinical Trial; FDA Clearance to QT Imaging to Calculate Fibroglandular Volume of the Breast; FDA 510k Clearance to iMediSync’s EEG scanner ‘iSyncWave’
Asahi Intecc partners with Penumbra to roll out Indigo System in Japan On September 22, 2022, Asahi Intecc, a Surgical and medical instrument manufacturing company, partnered with Penumbra, a medical device company headquartered in Alameda, California, to roll out Indigo System in Japan. After getting regulat...
Read More...
Aug 11, 2022
Rapid Medical’s TIGERTRIEVER 13; Glaukos’s Istent Infinite System; GE Healthcare’s Definium 656 HD; NeuroOne’s Signed Exclusive Development & Distribution Agreement with Zimmer; Kaia Health’s Rise-uP Randomized Controlled Trial; Bluejay’s Symphony IL-6 Test
Rapid Medical Obtains FDA Clearance for the World's Smallest and Only Adjustable Thrombectomy Device On July 26, 2022, Rapid Medical, a leading developer of advanced neurovascular devices, received Food and Drug Administration (FDA) 510(k) clearance for TIGERTRIEVER™13 for large vessel occlusions.&n...
Read More...



-Agonist.png)

