Thymidine Kinase 2 Deficiency Treatment: Advances in Therapeutic Strategies and Clinical Development
Jun 09, 2025
Table of Contents
Thymidine kinase 2 deficiency (TK2d) is a rare and life-threatening genetic condition and a type of mitochondrial disease. It stems from mutations in the TK2 gene, which is essential for the replication of mitochondrial DNA (mtDNA). These mutations lead to a depletion of mtDNA, resulting in profound muscle weakness and various systemic complications.
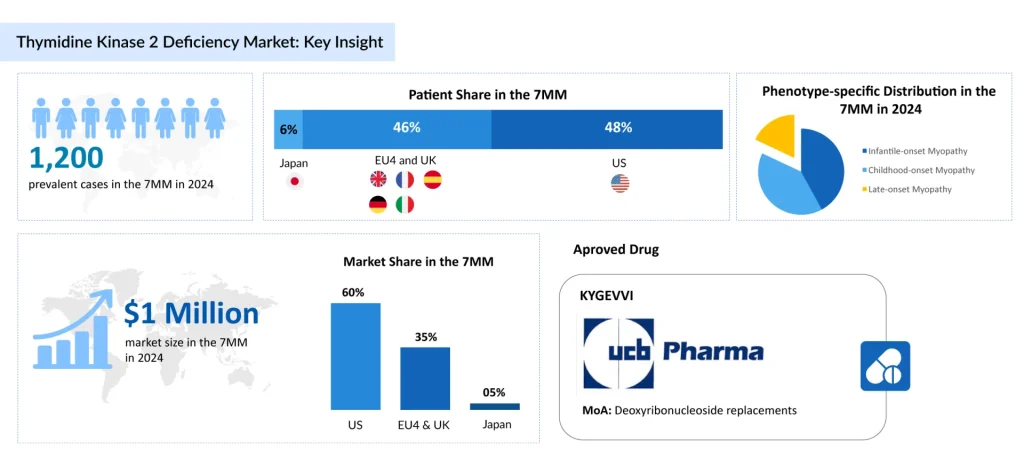
According to DelveInsight’s analysis, there were nearly 1,200 prevalent cases of TK2d across the 7MM in 2024. This number is projected to increase over the 2025–2034 forecast period, driven by improvements in genetic diagnostics and expanded access to healthcare.
Downloads
Click Here To Get the Article in PDF
Among the different phenotypes, infantile-onset myopathy represented about 40% of all diagnosed prevalent cases in 2024 across the 7MM, highlighting the significant burden posed by early-onset forms of the disease.
The rise in TK2d cases is anticipated during the forecast period (2025─2034) due to improved genetic testing, increased clinical awareness, and advancements in diagnostic technologies. As recognition of phenotype variability grows and healthcare systems enhance rare disease tracking, more cases, previously undiagnosed or misclassified, are likely to be identified, leading to a measurable increase in diagnosed prevalence across the 7MM.
Therapeutic Gaps in Thymidine Kinase 2 Deficiency Treatment
In the absence of approved therapies specifically targeting TK2d, treatment primarily revolves around managing symptoms through a coordinated, multidisciplinary approach. This approach plays a vital role in enhancing the quality of life and preventing complications. Neurological care is especially important, with physical and occupational therapy aimed at countering progressive muscle weakness, supporting mobility, and preserving independence in daily activities. As the disease advances, respiratory issues become more pronounced, often necessitating non-invasive or invasive ventilation to maintain adequate oxygen levels and prevent respiratory failure—an intervention crucial for extending survival and reducing respiratory strain.
Nutritional care is equally critical, as patients frequently struggle with malnutrition due to swallowing difficulties, fatigue, and muscle weakness. To meet energy demands, high-calorie diets are typically advised, and in severe cases, gastrostomy feeding may be required to ensure proper nutrition. As mobility declines, assistive devices such as wheelchairs become increasingly important in supporting independence and reducing physical limitations. Although these supportive measures are essential in managing TK2d, the absence of disease-specific therapies highlights a pressing need for treatments that directly target the underlying pathology.
Scarcity of Clinical Candidates in Thymidine Kinase 2 Deficiency Treatment
The TK2d treatment pipeline remains very limited, with only a handful of candidates progressing through clinical development. Among these, MT1621 from UCB Pharma is the leading emerging therapy anticipated to launch soon. Its expected approval is poised to fill a crucial therapeutic gap and significantly enhance patient outcomes.
MT1621 is a novel oral nucleoside therapy composed of doxecitine and doxribtimine, designed as a fixed-dose combination. It works by addressing the core mitochondrial dysfunction in TK2d patients, supplying thymidine and deoxycytidine to restore the accuracy of mitochondrial DNA replication and improve skeletal muscle function. Preclinical studies have shown that this treatment increases mtDNA copy numbers, balances nucleotides, and extends survival.
In March 2025, UCB presented promising results for doxecitine and doxribtimine at the MDA Conference. The data, drawn from clinical trials and an expanded access program, demonstrated significant improvements in survival and functional outcomes, particularly in patients with early symptom onset. Currently, the therapy is undergoing regulatory review in the US and EU but has yet to receive approval.
Back in February 2019, the US FDA granted Breakthrough Therapy Designation (BTD) to MT1621 for TK2d treatment. The therapy also received PRIME designation from the EMA and Orphan Drug Designation (ODD) from both the US FDA and EMA in 2018. MT1621 is presently being assessed in a Phase II clinical trial (NCT03701568) to determine its efficacy in managing TK2d.
Obstacles Hindering Progress in Thymidine Kinase 2 Deficiency Treatment
Thymidine kinase 2 deficiency treatment faces several significant roadblocks that hinder effective therapeutic development and patient outcomes. One major challenge is the rarity and genetic complexity of TK2d, which limits the availability of comprehensive clinical data and well-characterized patient populations for robust clinical trials. This scarcity complicates the identification and validation of effective treatment candidates. Additionally, the mitochondrial nature of TK2d presents unique hurdles, as delivering therapies that efficiently target mitochondrial dysfunction without off-target effects remains technically demanding. The biochemical pathways involved in TK2d are intricate, and therapies must precisely restore mitochondrial DNA replication without disrupting other cellular processes, adding another layer of complexity to drug development.
Another critical obstacle is the limited number of advanced therapeutic candidates progressing through TK2d clinical pipelines. While some promising molecules like MT1621 are emerging, many potential therapies remain in early-stage development, delaying broader patient access. Furthermore, the lack of standardized diagnostic tools and biomarkers to monitor disease progression and treatment response makes it challenging to evaluate therapeutic efficacy objectively. Economic and regulatory factors also play a role, as the high cost of developing treatments for rare diseases, coupled with stringent approval requirements, can discourage investment and slow innovation. Collectively, these roadblocks underscore the urgent need for collaborative research, improved diagnostic frameworks, and innovative delivery platforms to accelerate the development of safe and effective TK2d treatments.
What Lies Ahead in Thymidine Kinase 2 Deficiency Treatment?
The future of thymidine kinase 2 deficiency treatment holds promising potential as novel therapeutic approaches advance through clinical development. As per DelveInsight, the total market size of TK2d in the 7MM in 2024 was approximately USD 1 million and is anticipated to grow with a significant CAGR during the forecast period. Increasing prevalence pool and rising awareness about genetic tests for TK2d may increase market size in the coming years, which can be supplemented by the launch of new therapies.
Emerging therapies, such as MT1621 by UCB Pharma, are poised to transform the treatment landscape by offering first-in-class oral nucleoside analogs that directly target the underlying mitochondrial DNA depletion characteristic of TK2 deficiency. This shift from purely supportive care to targeted molecular intervention is expected to improve patient outcomes significantly by slowing disease progression and enhancing quality of life. Additionally, ongoing research into gene therapy and enzyme replacement strategies may further expand treatment options, providing hope for more durable and potentially curative solutions.
Despite these encouraging developments, challenges remain in fully addressing the heterogeneity and complexity of TK2 deficiency. The rarity of the disease limits large-scale clinical trials, making regulatory approval and widespread adoption slower. However, increasing awareness, improved diagnostic techniques, and collaborative efforts between researchers, clinicians, and industry stakeholders are accelerating progress.
As new treatments emerge, personalized medicine approaches tailored to individual patient genotypes and disease severity will likely become integral to TK2 deficiency management, ushering in a new era of precision therapies and improved prognosis for affected individuals.





