Ever-Evolving Market Landscape of Transcatheter Aortic Valve Replacement
Mar 29, 2021
Table of Contents
Transcatheter Aortic Valve Replacement (TAVR), also known as Transcatheter Aortic Valve Implantation (TAVI), is a minimally invasive surgery performed to insert a new valve without removing the damaged one. The narrowed aortic valve, which has failed to open properly (aortic valve stenosis), is replaced by a new one. Generally, a valve replacement requires an open-heart surgery with a sternotomy, where the chest is surgically opened for the procedure. TAVR is done through tiny openings without disturbing the chest bones. Aortic valve stenosis is the result of the heart’s aortic valve thickened and calcified, preventing the valve from opening fully and limiting the blood flow from the heart to the rest of the body. This can lead to heart failure and sudden cardiac deaths.
Transcatheter Aortic Valve Replacement: Types of surgery
- The transfemoral approach does not require a surgical incision in the chest and is performed through the femoral artery (large artery in the groin).
- The transapical approach requires a minimally invasive surgery with a small incision in the chest and entering through a large artery or through the tip of the left ventricle (the apex).
- Transaortic Implantation is an alternative to the transfemoral and transapical approaches and is suggested because it has a fast recovery rate and less mortality.
Eligible patients
TAVR procedure is performed in patients who have symptomatic severe aortic stenosis at low, intermediate, or high risk for standard valve replacement surgery. It is helpful for patients who have limited options for the repair of their aortic valve. The patients with aortic stenosis signs and symptoms are suggested to get the implant. With the help of TAVR, quality of life is improved in patients who otherwise don’t have many treatment options left.
Downloads
Click Here To Get the Article in PDF
Recent Articles
It was estimated that in 2017, around 7% of the population over the age of 65 years suffered from degenerative aortic stenosis. Further, over 276,000 patients underwent a TAVR procedure since its approval in 2011 in the United States. According to the data by the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) TVT Registry, TAVR volume (72,991) exceeded all forms of surgical aortic valve replacement (SAVR) volume (57,626) for the first time in 2019. In 2019, it was estimated that 56% of the males underwent TAVR in the United States.
Severe aortic stenosis that occurs after the aortic valve becomes diseased (stenotic) affects approximately more than 500,000 patients in Western Europe per year.
In 2019, Japan had a prevalence of 2.5% of any valve disease, out of which 0.4% was aortic valve stenosis. The elderly population is the most affected one, with a prevalence of 4.5% at the age of 75 years and 8.1% at 85 years.
Procedure
Transcatheter aortic valve replacement is made of a wire valve frame and bovine (cow) animal tissue leaflets and is designed to restore proper function to the diseased aortic valve. The surgeon will make a small incision in an artery or blood vessel, most commonly in the groin, and insert a small, hollow tube, called a catheter. The artificial valve is compressed and placed onto the catheter, and this will reach the diseased aortic valve through the blood vessel. With the help of advanced imaging techniques such as X-ray, the surgeon will push the diseased parts of the aortic valve leaflets out of the way and place the new valve. The new valve will start functioning once placed and restore the blood flow. Then, the surgeon will remove the catheter and close the incision. The patient will be transferred to the recovery department and will be discharged within days. Blood-thinning medications will be prescribed to prevent future blood clots.
What Risk Does TAVR Carry?
Transcatheter aortic valve replacement benefits the patients who are left with no alternative treatment. However, TAVR is almost always associated with risks. These include:-
- Excessive Bleeding
- Complications with blood vessel
- Errors with the replacement valve, such as the valve slipping out of place or leaking
- Higher chances of stroke
- Arrhythmias (heart rhythm problems) and the requirements for pacemaker implantation
- Heart attack
- Infection
- Kidney disease
- Fatality
Transcatheter Aortic Valve Replacement Market Dynamics
The Transcatheter Aortic Valve Replacement market is driven by the increased incidence of cardiovascular diseases and the increasing geriatric population. There has been a higher acceptance in adopting advanced surgical procedures that have led to an increase in the investments and funding in health care by both government and individuals. There is also an availability of financial assistance for patients getting TAVR to lower down the cost burden.
Even then, it is expected that the high cost of TAVR and risks like blood vessel complication, stroke, arrhythmias, infections, and others associated with TAVR procedure shall hinder the TAVR market growth. Like open-heart valve replacement surgery, treatment with transcatheter heart valves is associated with a potential risk of serious complications that include death, stroke, acute kidney injury, heart attack, bleeding, and the need for a permanent pacemaker.
Pharmaceutical Companies in the TAVR Market
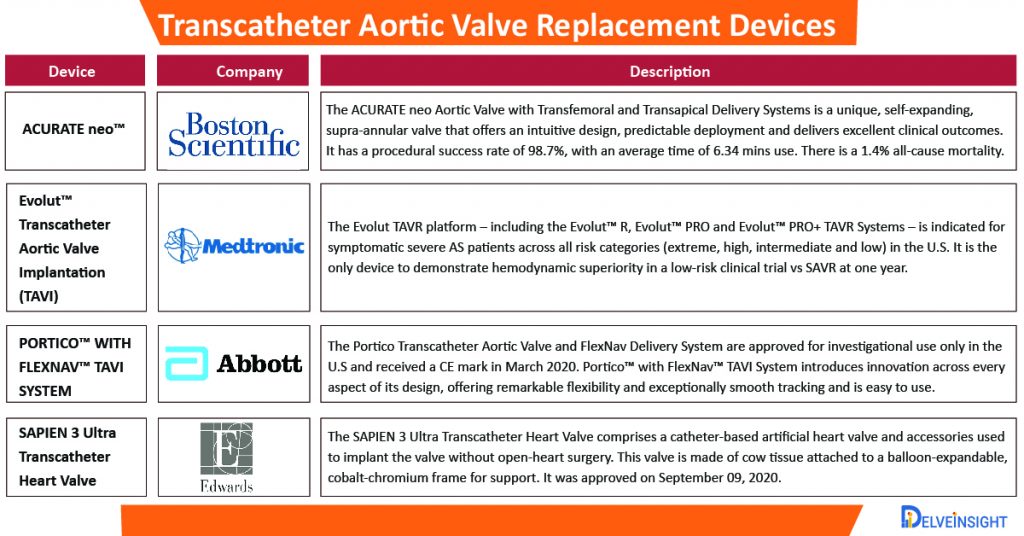
Several pharmaceutical companies operating in the Transcatheter Aortic Valve Replacement market include Medtronic plc, Boston Scientific Corporation, Edwards Lifesciences Corporation, Abbott, St. Jude Medical, Inc JenaValve Technology, Inc., Bracco SpA, Transcatheter Technologies GmbH, JC Medical, Inc., and NVT AG among others.
Key Developmental Activities in the TVAR Market
- On November 17, 2020, Boston Scientific Corporation announced the initiation of its global, voluntary recall of all unused inventory of the LOTUS Edge™ Aortic Valve System due to complexities associated with the product delivery system.
- In October 2020, Medtronic plc started SMall Annuli Randomized To Evolut™ or Sapien (SMART) post-market trial, which will compare valve safety and performance of the self-expanding Medtronic Evolut™ PRO and PRO+TAVR Systems against the balloon-expandable SAPIEN 3 and SAPIEN 3 Ultra Transcatheter Heart Valves manufactured by Edwards Lifesciences. It is expecting an enrolment of 700 patients globally.
- On September 09, 2020, the Food and Drug Administration (FDA) approved the premarket approval application (PMA) for the Edwards SAPIEN 3 and SAPIEN 3 Ultra Transcatheter Heart Valve System for expanding the indications to include patients with a failing transcatheter bioprosthetic aortic valve.
- On June 22, 2020, Medtronic plc announced the CE (Conformité Européenne) mark and European launch of the Evolut™ Transcatheter Aortic Valve Implantation system for patients with severe native aortic stenosis who are at low risk of surgical mortality.
- On March 05, 2020, Abbott received CE Mark for the new FlexNav™ delivery system for the company’s Portico™ transcatheter aortic valve implantation (TAVI) system, enabling marketing authorization in Europe.
- In August 2019, the U.S. Food and Drug Administration (FDA) opened the use of transcatheter aortic valve replacement (TAVR) to low-risk patients, expanding the indications for use for both the Edwards Lifesciences’ Sapien 3 valve and Medtronic’s CoreValve Evolut system for this patient population.
Conclusion
Aortic valve stenosis (AS) is the most common valvular pathology, which has been traditionally managed using surgical aortic valve replacement (SAVR). With the introduction of transcatheter aortic valve implantation (TAVI), there has been a transformation in the available healthcare. These improved outcomes are because of the combination of the simplification of the devices used, better designs to improve TAVR valve performance, and enhanced procedural performance as operators gain more experience. The minimum invasive procedure nature of TAVR holds future promise to become an out-patient procedure.
The Transcatheter Aortic Valve Replacement market is expected to see growth as the FDA expanded the indication for the use of TAVR to low-risk patients for death or have significant complications associated with open-heart surgery. In 2019, and this will take over as the new standard-of-care, replacing surgery in about 70 percent or more in the recent few years. The therapy has a very low mortality rate, has excellent outcomes, has very low stroke rates, and can even out-perform surgery.
Though, the market will be hindered because of lacks the long-term durability data that surgical valves have. There is not much information about the life of a TAVR valve before it wears out and needs to be replaced because of its relatively new technology nature, which makes some physicians hesitant about implanting these devices in younger patients.
Thus, there has been tremendous progress in treating severe aortic stenosis with TAVR, and there is room for improvements before it becomes the default therapy for all patients.
Downloads
Article in PDF
Recent Articles
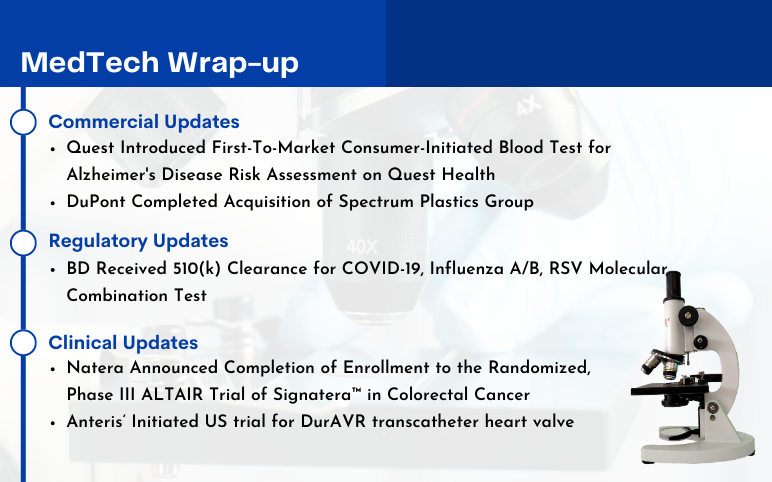
- What is Cholangiocarcinoma (CCA)?
- Edwards’s Pascal Precision System; Abbott’s New Spinal Cord Stimulation Device; Imagin to A...
- How Metaverse is Set to Transform the Healthcare Dynamics?
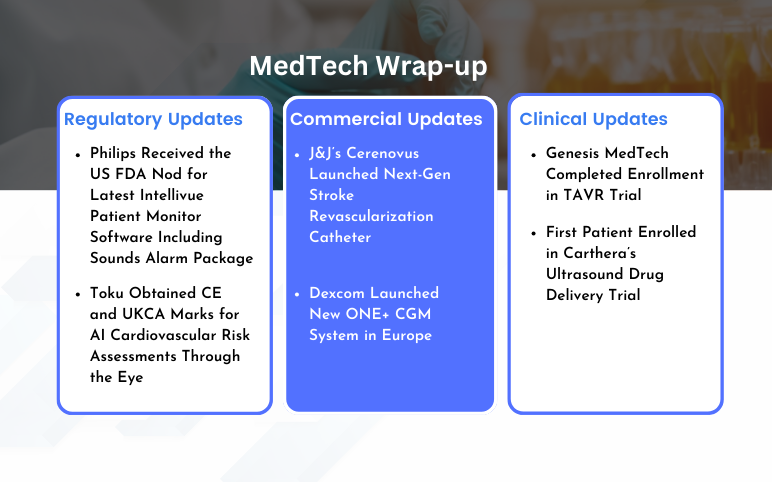
- First Patient Enrolled in Kaizen Clinical Study in Japan; Koneksa’s At-Home Mobile Spirometry Cli...
- Stryker’s Tornier Shoulder Arthroplasty; Genesis Acquires JC Medical; FDA Clearance to Single-Use...