Current and Emerging therapies that change Traveler’s Diarrhea Market
Apr 05, 2019
Traveler’s Diarrhea is an intestinal infection, a digestive tract disorder that requires research as globally many tourists are affected by it. According to the United Nations World Tourism Organization, in 2004, around 25%-50% of approximately 34 million international travelers, who visited developing countries, and tropical areas like Latin America, the Caribbean, the Middle East, South, and Southeast Asia, and Africa, from more developed or industrialized countries, are expected to experience Traveler’s Diarrhea (TD) due to the consumption of food or water contaminated with fecal.
According to DelveInsight’s estimates, the EU5 and, the US accounts for approximately 43% of the total Incident cases out of all the TD cases in 7 major markets in 2017. Among the EU5 countries, the highest % of diagnosed Traveler’s Diarrhea incident population was in Germany followed by Italy based on the recent studies and data from registries in 2017.
Although TD is not a fatal disease
Downloads
Click Here To Get the Article in PDF
Recent Articles
The Traveler’s Diarrhea Market
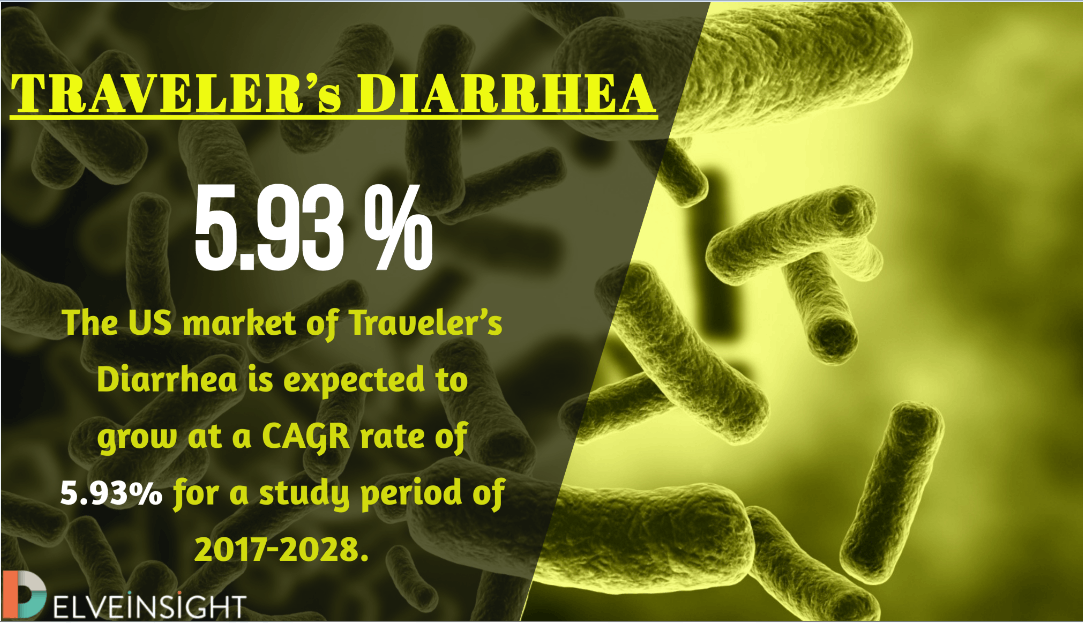
The total Traveler’s Diarrhea market size was USD 1,717 Million in 2017 for the major markets. DelveInsight’s analysts estimate that it is expected to show positive growth in the upcoming years. The total Traveler’s Diarrhea market is set to expand by 2028. The analysis shows that the US Traveler’s Diarrhea market is expected to grow at a CAGR of 5.93% for the study period. This accounts for almost doubling of Traveler’s Diarrhea market size by 2028 making it reach 3,106 Million by 2028.
The current US Traveler’s Diarrhea’s market is dominated by the use of antibiotics as they form the 1st line of treatment.
Antibiotics such as fluoroquinolones comprising ciprofloxacin, ofloxacin, norfloxacin, and levofloxacin. Antibiotic therapy is recommended either with or without loperamide for travelers with moderate to severe symptoms. Antibiotics, anti-motility drugs (for symptomatic relief) and Dukarol (Vaccine) are extensively being used as an off label therapy. The current market also holds a single approved therapy Xifaxan by Salix Pharmaceuticals. The second line of treatment i.e. azithromycin provides a potential alternative to fluoroquinolones. Azithromycin is considered as a treatment of choice for children between the ages of 2 and 8 years and for pregnant women, for whom the use of fluoroquinolones is contraindicated. The treatment of traveler’s diarrhea includes Oral Hydration as it replenishment fluids and electrolytes to prevent and treat dehydration. Also, the most common treatments include bismuth
Not to be missed, traveler’s Diarrhea pipeline has seen constant upgradations in past years. DelveInsight believes that the current market for Traveler’s Diarrhea will increase in the upcoming years with the emergence of several upcoming therapies. A significant shift is expected in the Traveler’s Diarrhea market because of the positive outcomes of several prevailing drugs by the companies, such as Scandinavian Biopharma, Procter and Gamble, etc. The Fast Track and QIDP designation holder Aemcolo/Relafalk by Cosmo Pharmaceuticals which is set to launch in 2019. The late-stage product Bismuth Subsalicylate has emerged as a promising option to enter the market in 2021 for Traveler’s Diarrhea. Another mid-stage product Etvax is a vaccine against diarrheal disease caused by ETEC expected to enter the market by 2023.
Trends in the Traveler’s Diarrhea Market have been changing over the past years. The emergence of new effective drugs will benefit the patients as earlier their options were limited.
Request for Sample Pages Traveler’s Diarrhea Market.
Downloads
Article in PDF