Vagus Nerve Stimulators Market Outlook
Jul 02, 2021
Table of Contents
Vagus nerve stimulators (VNS) is a neuromodulation therapy approved by the United States Food and Drug Administration (FDA) as a palliative treatment for patients with both epilepsy and depressions. Other non-FDA-approved uses of VNS have been studied and explored in various small open-labeled studies which include Alzheimer’s disease, bipolar disorders, chronic refractory headaches, treatment-resistant anxiety disorders, and obesity.
VNS are used to deliver electric impulses for stimulating the longest cranial nerve in the body known as the vagus nerve that has a major role in motor and sensory functions. There are two vagus nerves present on the left and right sides of the body.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Insulet Corporation’s Omnipod® 5 Automated Insulin Delivery System Receives FDA Approval; FDA Giv...
- FDA Clears Fujirebio’s Lumipulse® G Plasma Test for Detecting Alzheimer’s-Linked Amyloid Patholog...
- Fluidx Medical’s GPX Embolic Device; Cardiovascular Systems & OrbusNeich’s Scoreflex S...
- eCential Robotics’s Surgical Robotic Platform for Spine Surgery; Baxter Gets 510(k) Clearance for...
- Ears to the Future: Evaluating Key Advancements and Trends Driving the Audiology Devices Market G...
A VNS is a small pulse-generating medical device generally implanted into the chest for the transmission of electrical impulses to the brain through the vagus nerve. These stimulators predominantly comprise three parts: a pulse generator, leads, and a handheld magnet. The pulse generator most often is a flat, round piece of metal accommodated with a miniature-sized battery. The generator is placed in the chest area under the skin through surgery and is programmed to send electrical impulses to the vagus nerve at regular intervals, all day, every day. The leads run from the generator to the vagus nerve in the neck and carry electrical signals to the vagus nerve while the hand-held magnet in some devices can be swept over the generator to send more impulses to the vagus nerve. Moreover, the programming software model allows communication with the pulse generator and can be programmed according to the need of the patients. There are majorly three types of approaches taken for stimulating the vagus nerve- Left cervical VNS, Right cervical VNS, and Transcutaneous forms of VNS.
Types of Vagus Nerve Stimulators
US FDA has approved mainly two types of vagus nerve stimulators. These are implantable (invasive) VNS devices and external (non-invasive) VNS devices.
- Invasive Vagus Nerve Stimulator
An invasive vagus nerve stimulator consists of a pulse generator, a lead, and in some cases a programming software. The VNS is a flat and round device made of metal. The device measures an inch and a half (approximately 4 centimeters) across and around 10-13 mm in thickness. Implantation of the VNS device is usually done as an outpatient procedure and requires two small incisions for placement of the device beneath the skin near the chest area. The first incision is done near the upper left side of the chest where the generator is implanted into a little “pouch” situated under the collarbone. To access the vagus nerve situated near the neck, the second incision is created by the surgeons. The leads are then wrapped around the left branch of the vagus nerve and the electrodes are connected to the generator. Once the stimulator is implanted successfully, the generator transmits electric impulses to the vagus nerve at regular intervals. Instead of the right vagus nerve, the left vagus nerve is stimulated because the right nerve has a major role in cardiac function, and stimulating it could have negative cardiac effects.
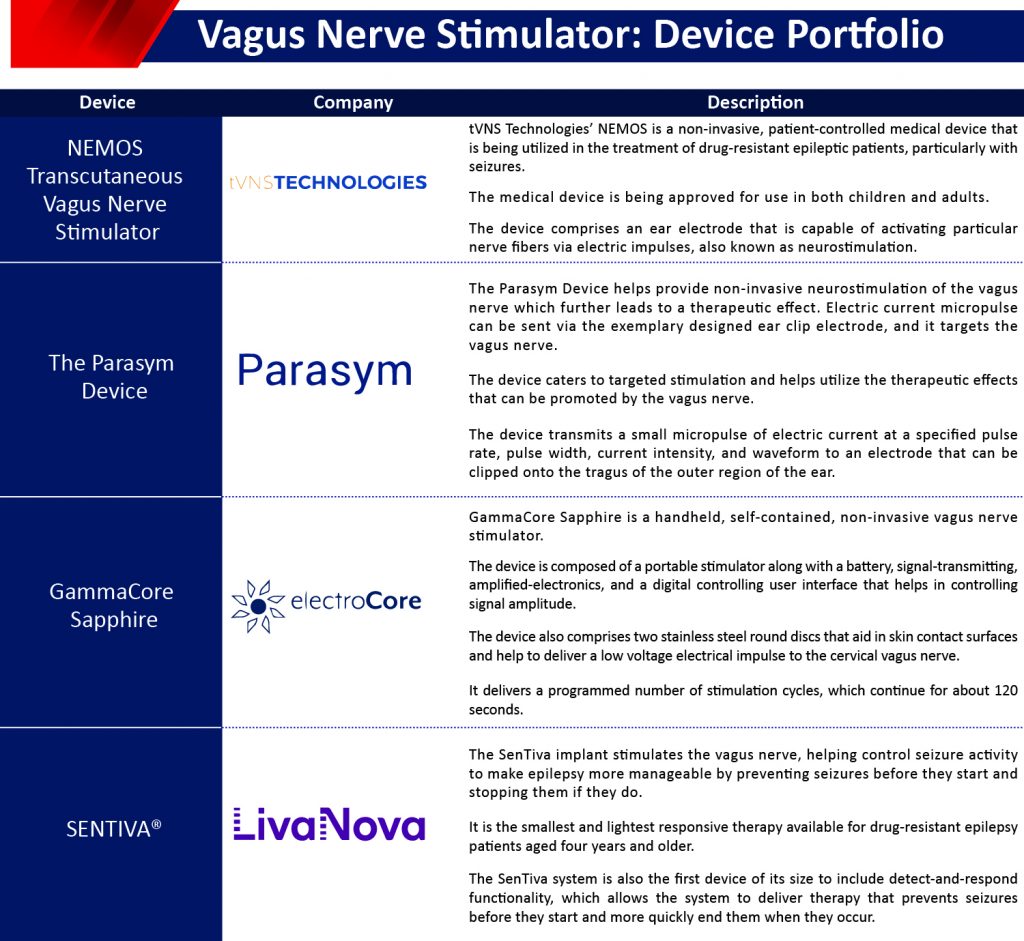
An example of invasive VNS devices is Symmetry Vagus Nerve Stimulator by Livanova available as a small device that works by regularly transmitting micropulse via a thin wire to the vagus nerve in the neck region. Other examples include SENTIVA® (Livanova), ReShape™ vBloc therapy (ReShape Lifesciences), and NeuroPace RNS System (NeuroPace) among others.
- Non-invasive Vagus Nerve Stimulator
Non-invasive or external vagus nerve stimulators are not implanted inside the body rather these are small either hand-held devices that transmit electrical impulses through surface or skin contacts. These devices sometimes comprise an ear electrode that is capable of activating particular nerve fibers via electric impulses, also known as neurostimulation.
These devices are mainly based on transcutaneous vagus nerve stimulation technology. gammaCore™ is a non-invasive vagus nerve stimulator that has been approved by the US FDA. The device is also approved in the European region. Some of the non-invasive devices present in the Vagus nerve stimulator Market are VITOS (tVNS Technologies), Parasym Device (Parasym Ltd.), and Transcutaneous Neurostimulator (Soterix Medical), among others.
Advantages of Vagus Nerve Stimulator
- Reduction in the frequency of seizures and less medication with anti-seizure drugs.
- Improvement in quality of life.
- Better recovery rate.
- By stimulating the vagus nerve, one can relax and de-stress at any time, improving the mood and wellbeing of patients.
- Better management of anxiety and depression.
- Cost-effective when compared to drugs.
Disadvantages of Vagus Nerve Stimulator
- There are chances of infection after a surgical procedure in around 3% to 6% of the patients. These infections are generally treated with oral antibiotics.
- Some patients are also left with left cord paralysis as the device operated in a continuous direct-current high-output mode cause pain, hoarseness, increased coughing, and change in voice.
- Due to the high surgical incisions, some patients also witness lower facial weakness.
- There is an increased risk of bradycardia (lower heart rate than usual) and asystole (cardiac arrest).
Therapy and its Usage
Vagus nerve stimulation therapy is being used since the 1880s as a manual massage and compression to suppress seizures. Since then many VNS devices have been approved by the FDA for the treatment of refractory epilepsy and depression. Additionally, vagus nerve stimulation provides a potential treatment option for a variety of conditions. Some of them are entailed below:
- Epilepsy
Epilepsy is a neurological disorder that is associated with seizures and result in involuntary movements of different extremities due to the abnormal electrical discharge in the brain. VNS therapy was first approved for the treatment of drug-resistant epilepsy. In 1997, FDA has approved VNS for seizure treatment among those patients who have shown no effective response to two or more anti-epileptic drugs and also have no sufficient control over their seizures. VNS is used simultaneously with anti-epileptic drugs (AEDs) and is not an alternative treatment. The treatment does not aim to completely eradicate seizures but is used in combination with AEDs as an alternative treatment for ameliorating seizure control.
- Depression
Depression is a mental disorder characterized by sadness, loss of interest in normal daily activities, feelings of dejection and hopelessness, and diminished ability to experience pleasure. According to World Health Organization Global Health Estimates, 2017 the proportion of the global population with depression in 2015 is estimated to be 4.4% which is approximately 322 million. Depression is more common among females (5.1%) than males (3.6%). Most of the depressive episodes are treatable with medications, however, roughly 30% of the population with major depression are less likely to respond to the medication. Thus, among individuals with chronic or recurrent treatment-resistant depression (TRD) and those who have failed to respond to four or more adequate treatments, Vagus nerve stimulation (VNS) clinically has shown to be efficacious for the long-term management of patients with treatment-resistant depression (Aaronson et al., 2017).
- Migraine
Migraine is a neurologic syndrome accompanied by recurrent episodes of painful, usually unilateral headaches and nausea. Clinical studies showed that VNS reduces the pain level of a migraine at 30 minutes and 60 minutes after using the device.
- Alzheimer’s
Alzheimer’s disease is the most common type of dementia and is a progressive disease that damages brain cells and destroys memory and other important mental functions. The disease is characterized by the loss of cognitive functions. Clinical evidence has proved VNS therapy to be effective in the form of motor speed, psychomotor function, language, and executive functions after 10 weeks of stimulation as it activates the degenerated and damaged regions of the brain.
- Heart failure
Chronic heart failure (HF) is linked with autonomic dysregulation. It is characterized by an increase in sympathetic drive and by the withdrawal of parasympathetic activity. There is an increased heart rate in patients and results in increased mortality and morbidity in myocardial infarction and HF. Studies showed an increase in long-term survival rates in the rat with VNS therapy.
- Obesity
Obesity resulted due to the ingestion of calories above the normal biological requirement is a major risk for a variety of chronic diseases, including cardiovascular disease, diabetes mellitus, chronic kidney disease, gallbladder diseases, and others. Obesity treatment can impose an enormous economic burden on the global healthcare system. VNS can be a potent tool for the treatment of obesity.
Eligible Patient Population
Epilepsy is the most common serious neurological disorder with the prevalence ranging from 0.5% to 1% of the populations in developed nations. The prevalence is even higher in developing countries. This disorder is characterized by repetitive spontaneous seizures. VNS therapy is a unique epilepsy treatment for those who do not respond to medications. Also, it has been a beneficiary treatment for TRD (treatment resistant depression) patients.
According to World Health Organization 2019 report, epilepsy accounts for a significant proportion of the world’s disease burden, affecting around 50 million people worldwide. The estimated proportion of the general population with active epilepsy at a given time is between 4 and 10 per 1000 people.
As per the Center for Disease Control & Prevention, in 2015, 1.2% of the US population had active epilepsy which is about 3.4 million people with epilepsy nationwide: 3 million adults and 470,000 children.
According to the study Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease published in Lancet, in 2019, Western Europe had a prevalence of 1,525,168 for idiopathic epilepsy with Germany having the maximum prevalent cases followed by France among EU 5 and Spain had the least number of prevalent cases. Also, as per the report, Japan had a prevalence of 247,249 for idiopathic epilepsy (Beghi et al., 2019).
In the year 2017, a total of 83,212 patients of DRE and 267,887 patients of TRD were eligible for VNS Device in seven major markets, which are anticipated to increase by the year 2025. The United States accounts for the highest eligible patient population of DRE and TRD.
Emerging Vagus Nerve Stimulator Therapies
The tVNS is a potential treatment that is not limited to the treatment of depression and epilepsy. This non-invasive advanced technology is being investigated for a variety of disorders including headache, tinnitus, atrial fibrillation, post-error slowing, prosocial behavior, associative memory, schizophrenia, and pain (Yap et al., 2020).
The invasive vagus nerve stimulators are the most prominent devices for VNS therapy. However, non-invasive VNS (nVNS) has been extensively researched as an alternative to invasive vagus nerve stimulators. nVNS does not require implantation surgery. These devices provide stimulation that works trans-cutaneously. nVNS devices either involve an intra-auricular electrode (NEMOS, Cerbomed, Erlangen, Germany) or a portable stimulator and digital user interface that controls signal amplitude (gammaCore, electroCore LLC, Basking Ridge, NJ, USA). NVNS has an advantage compared to conventional VNS as it does not require a surgical implantation procedure, thus minimizing the risk of infection. Stefan et al. had stimulated the left auricular branch of the vagus nerve in 10 adult patients and noticed that the seizure frequency was reduced by 45% and 48% in two patients. He et al., another researcher also investigated the outcome of transcutaneous VNS and showed a mean reduction seizure frequency of 54% at 6 months.
Vagus Nerve Stimulator Market Activity
- In September 2020, NeuroSigma received Bridge Financing from Checkmate Capital to support the targeted commercial launch in the US of its patented Monarch® eTNS® System to treat pediatric ADHD.
- In March 2020, NeuroPace announced FDA approval of MRI labeling for its RNS system for people living with seizures that do not respond to medication.
- In March 2020, Electrocore LLC has received FDA clearance as a prevention treatment of migraine headaches in adult patients. In February 2021, the FDA expanded the clearance of GammaCore (by Electrocore LLC) to include the acute and preventive treatment of migraines in adolescents between ages 12 and 17.
- On February 26, 2020, LivaNova had entered into a research collaboration with Verily to increase their understanding of Vagus Nerve Stimulators and their impact on Difficult-to-Treat Depression. Verily is capturing measures of Depression with the RECOVER clinical study and is evaluating the effectiveness of the Vagus Nerve Stimulator for the treatment of difficult-to-treat epilepsy.
- In April 2019, The U.S. Food and Drug Administration permitted the use of the first medical device (Monarch eTNS System) by NeuroSigma to treat attention deficit hyperactivity disorder (ADHD) in the Vagus nerve stimulator therapy market.
- In April 2018, LivaNova Received CE Mark for VNS Therapy SenTiva Generator and Next-Generation Programming System for Treatment of Epilepsy.
Vagus Nerve Stimulator Market: Current and Future Scenario
The vagus nerve stimulator devices have brought about a revolution in the treatment of drug-resistant epilepsy, and major depressive episodes of TRD. These devices have significantly improved the patient’s outcome. Various invasive vagus nerve therapy stimulators are being clinically investigated and are being developed at a faster pace due to their benefits in treating an increasing population suffering from epilepsy and depression who are resistant to drugs. Additionally, due to robust R&D, these devices are also being investigated for other chronic disorders such as diabetes, heart failure, obesity, Alzheimer’s in various clinical trials which is likely to augment the Vagus nerve stimulator market. These devices are gaining popularity due to their small size and extended battery life of ten years. In patients with drug-resistant epilepsy and treatment-resistant depression, VNS has been a potential beneficiary device. Once the device is on it automatically reduces the seizures and does not require continuous patient involvement. Another potential advantage is that swiping a magnet across the device releases an urgent electrical stimulation and gives the individual some control particularly when there is a cluster of seizures or an acute seizure occurring. Though VNS is not a cure it can improve the quality of life. As compared to medications, it has a better recovery rate, shows a prompt reduction in the frequency of seizures, and are cost-effective. These are the factors that are anticipated to increase the demand for VNS in the projected year.
The emergency use authorization granted by the U.S. Food & Drug Administration for treating suspected COVID-19 patients with a hand-held non-invasive vagus nerve stimulator the gammaCore Sapphire CV device by electroCore is likely to spur in the Vagus nerve stimulator market during the current COVID-19 pandemic scenario. According to FDA’s authorization, the device can be used either at home or in a clinic or hospital to acutely treat adult patients with known or suspected COVID-19 who are experiencing an exacerbation of asthma-related [shortness of breath] and reduced airflow, and for whom approved drug therapies are not tolerated or provide insufficient symptom relief. During the Covid-19 national emergency, many clinical trials are ongoing for exploring the VNS devices. One such clinical trial with NCT04379037 is sponsored by Nemechek Technologies.
However, owing to some complications associated with implantable stimulators such as general pain, indigestion, increased coughing, hoarseness in voice, nausea or vomiting, and others., along with the high cost of the implantable VNS devices and their surgical procedure have paved the way for non-invasive stimulators with novel transcutaneous technology that provides stimulation to the auricular branch of the vagus nerve are being extensively studied under clinical trials and are being developed at a faster pace. Moreover, shifting preferences for non-invasive devices among patients associated with migraine, depression, and epilepsy and limited availability of non-invasive marketed products have a high tendency to escalate the market for noninvasive devices in the coming future along with invasive devices.
As per Delveinsight’s analyst, the Vagus nerve stimulator market is expected to surge in the future due to the increasing incidence of neurological conditions, the resistance of patients to epileptic drugs or anti-depressants, technological advancements, and increasing demand for innovative devices such as NEMOS and VITOS among many others. These devices are expected to likely increase the market for vagus nerve stimulators during the forecasted period due to their many benefits along with the invasive stimulators which are dominating the VNS market and are anticipated to continue their dominance during the forecasted period.
Downloads
Article in PDF
Recent Articles
- BioGX’s ‘pixl’ Portable qPCR Platform; Stryker’s Citrefix Suture Anchor System; Boston Scientific...
- How Are Innovations in Drug Delivery Devices Solving the Complex Challenges in the Market?
- Guard Medical Announced FDA 510(k) Clearance; PROCEPT BioRobotics Announced FDA Clearance; GE Hea...
- Ears to the Future: Evaluating Key Advancements and Trends Driving the Audiology Devices Market G...
- Agiliti Launches Essentia; KUBTEC Introduces PICASSO Plus; FDA Clears Neuvotion’s NeuStim™ for No...



