5 Novel CDK7 Inhibitors Pushing the Boundaries in Oncology
Nov 11, 2024
Table of Contents
In the rapidly evolving field of oncology, novel CDK7 inhibitors are emerging as game-changers, challenging the status quo of cancer treatment. By specifically targeting CDK7, these innovative therapies not only halt cancer cell proliferation but also enhance the effectiveness of existing treatments. With the ability to modulate transcription and cell cycle progression, CDK7 inhibitors are poised to overcome resistance mechanisms that often hinder traditional therapies, offering renewed hope for patients battling aggressive malignancies.
Moreover, the versatility of CDK7 inhibitors positions them as pivotal players in combination therapies, synergizing with other agents to amplify anti-tumor effects. As clinical trials continue to unveil their potential, the integration of CDK7 inhibitors into standard treatment regimens could herald a new era of precision oncology. This exciting frontier promises to redefine treatment protocols, tailor strategies to individual patient profiles, and ultimately improve outcomes for those facing the daunting challenge of cancer.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- New biomarkers for screening in Pancreatic Cancer
- Incyte meets endpoint in second atopic dermatitis trial; NeoTX lands USD 45 M series C; Five Prim...
- AstraZeneca’s Danicopan Trial; CHMP Recommends Sanofi/AstraZeneca’s nirsevimab; Akero’s NASH Drug...
- Pancreatic Cancer Treatment to Stride Beyond Chemotherapy
- New biomarkers improve standard screening in Pancreatic Cancer
Discover more about CDK7 inhibitors at CDK7 Inhibitors: A Promising Class in Cancer Therapeutics
Emerging CDK7 Inhibitors in Development
For more than ten years, there has been a consistent interest in targeting CDK7 as a therapeutic strategy, with notable advancements occurring recently. The field of CDK7 inhibitors is expanding quickly, featuring various chemical classes that show promising kinase selectivity. These inhibitors function through multiple mechanisms, such as traditional competitive inhibition, irreversible binding, and selective degradation of CDK7 using heterobifunctional agents.
Both preclinical and clinical evidence highlight the potential benefits of CDK7 inhibition, especially in advanced HR+/HER2− breast cancer cases that have progressed despite the use of CDK4/6 inhibitors and hormonal therapies. While no CDK7 inhibitor has yet gained FDA approval, current clinical trials are focused on improving its effectiveness. Major companies developing CDK7 inhibitor drugs in clinical trials include Carrick Therapeutics (Samuraciclib), Qurient Therapeutics (Q901), Syros Pharmaceuticals (SY-5609), TYK Medicine (TY-2699a), and GT Apeiron/Exscientia (GTAEXS617).
Let’s take a look at the 5 most promising CDK7 inhibitors poised to reshape the oncology treatment landscape in the coming years.
Carrick Therapeutics’ Samuraciclib
Samuraciclib is the most advanced oral CDK7 inhibitor currently in clinical development. Its primary target is HR+, HER2—breast cancer, with additional potential for treating a range of other cancer types. Early clinical trials have shown samuraciclib to have a favorable safety profile and promising efficacy.
The FDA has granted Fast Track designation to samuraciclib for two combination therapies: with fulvestrant for CDK4/6 inhibitor-resistant HR+, HER2- advanced breast cancer, and with chemotherapy for treating locally advanced or metastatic triple-negative breast cancer.
In December 2023, the company announced the dosing of the first patient in its Phase IIb clinical trial assessing samuraciclib (CT7001) combined with fulvestrant for HR+, HER2- advanced breast cancer previously treated with a CDK4/6 inhibitor.
Qurient Therapeutics’ Q901
Q901 is a potent and selective CDK7 inhibitor that interferes with tumor cell cycle progression and impedes DNA damage repair, leading to tumor cell apoptosis. By inducing genomic instability, Q901 not only drives cell death but also enhances immune surveillance against tumor cells. As the company’s second oncology drug candidate, Q901 is being developed to treat patients with advanced solid tumors. It has shown robust tumor growth inhibition across several cancer types, including HR+ breast cancer, pancreatic, prostate, ovarian, and small cell lung cancers, both as a single agent and in combination with other therapies.
Currently, Q901 combined with fulvestrant is in clinical trials for HR+ breast cancer patients who have not responded to CDK4/6 inhibitor and estrogen therapies. A Phase I/II study (NCT05394103) is ongoing at six sites in the US, with approximately 70 patients with advanced solid tumors set to be enrolled. This study aims to establish the maximum tolerated dose, assess the safety profile, and evaluate the anticancer efficacy of Q901.
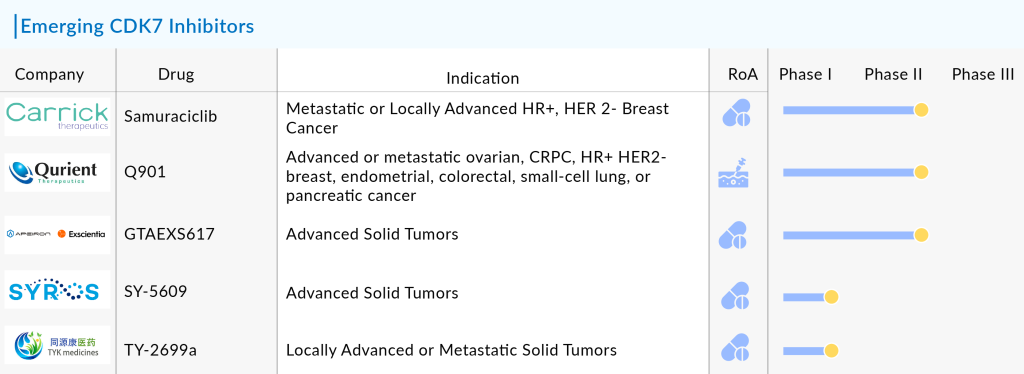
GT Apeiron/Exscientia’ GTAEXS617
GTAEXS-617 is a novel CDK7 inhibitor developed by Exscientia in collaboration with GT Apeiron. This small-molecule antagonist has been designed using artificial intelligence to achieve high potency, selectivity, and oral bioavailability.
In July 2024, Apeiron Therapeutics announced a partnership with Exscientia, which has been granted complete control over GTAEXS617 (‘617) and all associated intellectual property. This asset is currently undergoing the ELUCIDATE Phase I/II clinical trials.
According to the agreement, Apeiron will receive an initial payment of $30 million in cash and equity, along with single-digit royalties if Exscientia or a third party commercializes ‘617, potentially exceeding $100 million in the coming years.
The ELUCIDATE trial’s dose escalation phase is focused on assessing the safety, pharmacokinetics, and pharmacodynamics of ‘617 in patients with advanced solid tumors, with recruitment for the trial progressing well. Data from this monotherapy phase is anticipated in the latter half of 2024, with a transition to a combination dose escalation phase planned for late 2024 or early 2025.
Syros Pharmaceuticals’ SY-5609
SY-5609 is a potent and highly selective oral inhibitor targeting cyclin-dependent kinase 7. The company has completed patient enrollment in the safety lead-in phase of the Phase I trial of SY-5609 in combination with chemotherapy for pancreatic cancer. In September 2022, the U.S. FDA granted Orphan Drug designation to SY-5609 for pancreatic cancer treatment. Additionally, SY-5609 is being assessed in combination with atezolizumab for patients with BRAF-mutant colorectal cancer as part of the Phase I/Ib INTRINSIC trial.
TYK Medicine’s TY-2699a
TY-2699a is an innovative small molecule kinase inhibitor developed independently by TYK Medicines, Inc. It is designed to selectively inhibit CDK7 and has demonstrated good oral bioavailability. This drug is intended for treating various cancers, including breast, ovarian, and other solid tumors, as well as blood cancers. Preclinical studies on pharmacokinetics and safety have shown TY-2699a to have promising drug-like and safety characteristics. In February 2023, TY-2699a received the SMP letter from the FDA, and by May 2023, it had also secured clinical trial notice approval from the CDE. Notably, TY-2699a is the first CDK7 inhibitor from China to receive IND clearance from both the FDA and CDE.
What Lies Ahead for CDK7 Inhibitors?
CDK7 inhibitors have emerged as a promising class of drugs in oncology, driven by their potential to disrupt critical cell cycle processes in cancer cells. CDK7 plays a central role in transcription and cell cycle regulation, particularly by activating other CDKs that drive cell proliferation. Many cancers exhibit dysregulated CDK7 activity, making it a strategic target for drug development.
Early studies and clinical trials have shown that CDK7 inhibitors can effectively reduce tumor growth, especially in cancers with high transcriptional dependencies, such as breast, ovarian, and certain hematologic cancers. Researchers are now exploring combinations of CDK7 inhibitors with other cancer therapies, such as immune checkpoint inhibitors or hormone therapies, to enhance therapeutic outcomes and potentially reduce resistance to monotherapies.
However, the future of CDK7 inhibitors is not without challenges. Selectivity and toxicity remain primary concerns, as off-target effects on other kinases can lead to adverse side effects. Researchers are also grappling with identifying patient populations most likely to benefit from these treatments, as not all tumors exhibit CDK7 dependency. Biomarker-driven studies are expected to help narrow down patient selection and improve treatment specificity.
Ongoing and future clinical trials are crucial to determine optimal dosing, safety profiles, and combination strategies for CDK7 inhibitors. Despite these hurdles, the potential of CDK7 inhibitors to target transcriptional addiction in cancer cells offers a unique therapeutic angle that may expand treatment options in oncology, particularly for aggressive and treatment-resistant tumors.

Frequently Asked Questions
CDK7 (Cyclin-Dependent Kinase 7) inhibitors are small molecules that target CDK7, a kinase involved in transcription regulation and cell cycle control. By inhibiting CDK7, these agents aim to halt cancer cell proliferation, especially in tumors with high transcriptional dependency or resistance to conventional therapies.
Some of the key CDK7 inhibitors under development include: Samuraciclib by Carrick Therapeutics, targeting HR+/HER2- breast cancer and triple-negative breast cancer. Q901 from Qurient Therapeutics, being evaluated in advanced solid tumors and in combination with fulvestrant for HR+ breast cancer. GTAEXS617 (GT Apeiron/Exscientia), in Phase I/II trials for advanced solid tumors. SY-5609 (Syros Pharmaceuticals), in early trials, including combination studies with chemotherapy or immunotherapy. TY-2699a by TYK Medicine, showing promise in both solid and hematologic tumors; also notable for being among the first from China to get IND approvals from both U.S. FDA and Chinese authorities.
None of the CDK7 inhibitors have been approved yet. The most advanced agent is Samuraciclib, now in Phase IIb trials in combination with fulvestrant for HR+/HER2- breast cancer after CDK4/6 inhibitor resistance. Others like Q901 and GTAEXS617 are in earlier Phase I/II trials exploring safety, dosing, and preliminary efficacy.
These inhibitors are being evaluated in several cancer types, especially those with high transcriptional demands. Examples include hormone receptor-positive (HR+) breast cancer (including patients resistant to CDK4/6 inhibitors), triple-negative breast cancer, pancreatic, ovarian, prostate, small cell lung cancer, and other advanced solid tumors.
Key barriers include ensuring selectivity (avoiding off-target effects on other kinases), managing toxicity, and identifying which patient populations will derive sufficient benefit (i.e., biomarker driven stratification). Also, optimizing combination regimens with existing therapies and determining ideal dosing and safety in larger populations are necessary before wide adoption.
Downloads
Article in PDF
Recent Articles
- Eylea HD Injection 8 Mg Approved By FDA; Veopoz Receives FDA Approval for CHAPLE Disease Treatmen...
- Treatment to Clinical Development – A Bumpy Journey for Pancreatic Cancer
- AstraZeneca’s Danicopan Trial; CHMP Recommends Sanofi/AstraZeneca’s nirsevimab; Akero’s NASH Drug...
- FDA Approves PENBRAYA for Most Common Serogroups Causing Meningococcal Disease; BIMZELX Approved ...
- How CDK4/6 Inhibitors Are Changing the Cancer Treatment Paradigm?