Emerging Lupus Nephritis Therapies: Addressing Unmet Needs through Innovation and Mechanistic Targeting
May 05, 2025
Lupus nephritis is a serious and common complication of systemic lupus erythematosus (SLE), characterized by inflammation of the kidneys that can lead to progressive organ damage and increased mortality. Approximately one-third of adults with SLE present with lupus nephritis at diagnosis, and up to half will develop it over time. Often asymptomatic in its early stages, lupus nephritis may eventually manifest with edema, changes in urine output, and elevated blood pressure—signs of impaired renal function. Early detection and timely intervention are critical to preserving kidney health and improving long-term outcomes.
Lupus nephritis affects 1.7 million people globally, with an estimated 390,000 diagnosed lupus nephritis cases across the 7MM as of 2024, according to DelveInsight, nearly half of which are in the United States. The disease predominantly affects women and is typically present between the ages of 20 and 40. The ISN/RPS classification system defines lupus nephritis into six histological classes, with Class III (~30%), Class IV (~40%), and Class V (~20%) being the most prevalent. This classification is central to guiding treatment decisions and assessing disease severity.
Current treatment strategies for lupus nephritis primarily focus on suppressing the overactive immune system to preserve kidney function and prevent progression to end-stage kidney disease (ESKD), which can affect up to 30% of patients within 15 years if left untreated. Standard regimens typically involve corticosteroids in combination with immunosuppressants such as mycophenolate mofetil (MMF), cyclophosphamide, azathioprine, and calcineurin inhibitors. While effective, these treatments are associated with significant risks, including infections, organ toxicity, and long-term metabolic complications.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- FDA Approves Genentech’s GAZYVA for Lupus Nephritis; Glaukos’ EPIOXA Wins FDA Approval for Kerato...
- Roche’s Gazyva receives FDA Breakthrough designation for Lupus nephritis
- Daiichi Sankyo’s Intravenous Iron Replacement Therapy; ANeuroTech’s Adjunctive Anti-depression Dr...
- Aurinia’s Lupkynis for Lupus; FDA Fast Track Designation for Toripalimab; Wren Therapeutics...
- CAR-T Beyond Cancer: Resetting Immunity in Autoimmune Diseases
Two targeted lupus nephritis therapies—BENLYSTA (belimumab), a B-cell inhibitor available in both intravenous and subcutaneous formulations, and LUPKYNIS (voclosporin), the first oral lupus nephritis-specific treatment—have expanded the therapeutic arsenal. When combined with traditional immunosuppressants, they offer improved renal remission rates and a steroid-sparing effect. Treatment approaches are increasingly personalized, guided by histological class and disease severity.
The 2024 ACR guidelines underscore a paradigm shift toward more aggressive and targeted management of Class III/IV and Class V lupus nephritis. They recommend a triple therapy approach comprising glucocorticoids with one of the following combinations: MMF plus belimumab, MMF plus a CNI, or low-dose cyclophosphamide plus belimumab. The guidelines advocate for pulse steroid initiation followed by rapid tapering to reduce glucocorticoid toxicity. Despite these advances, complete renal response (CRR) rates remain suboptimal at 40–50%, and treatment duration is typically extended over 3–5 years to maintain disease control.
Despite these advances, significant unmet needs persist. Up to 50% of SLE patients are not routinely screened for kidney involvement, and 77% of those with lupus nephritis remain untreated. Current therapies often fail to induce complete remission—fewer than 60% achieve this after two years—with high relapse and progression to ESKD in up to one-third of patients within a decade. Long-term steroid use remains a major contributor to morbidity. These gaps highlight the urgent need for novel therapies that enhance efficacy, minimize toxicity, and deliver durable disease control.
The emerging pipeline for lupus nephritis treatment reflects an increasingly diversified approach. Several late- and early-stage candidates are in development, including ianalumab (VAY736), obinutuzumab (GAZYVA), SAPHNELO (anifrolumab), FABHALTA (iptacopan), ULTOMIRIS (ravulizumab), and cutting-edge cellular therapies such as CABA-201 and YTB323.
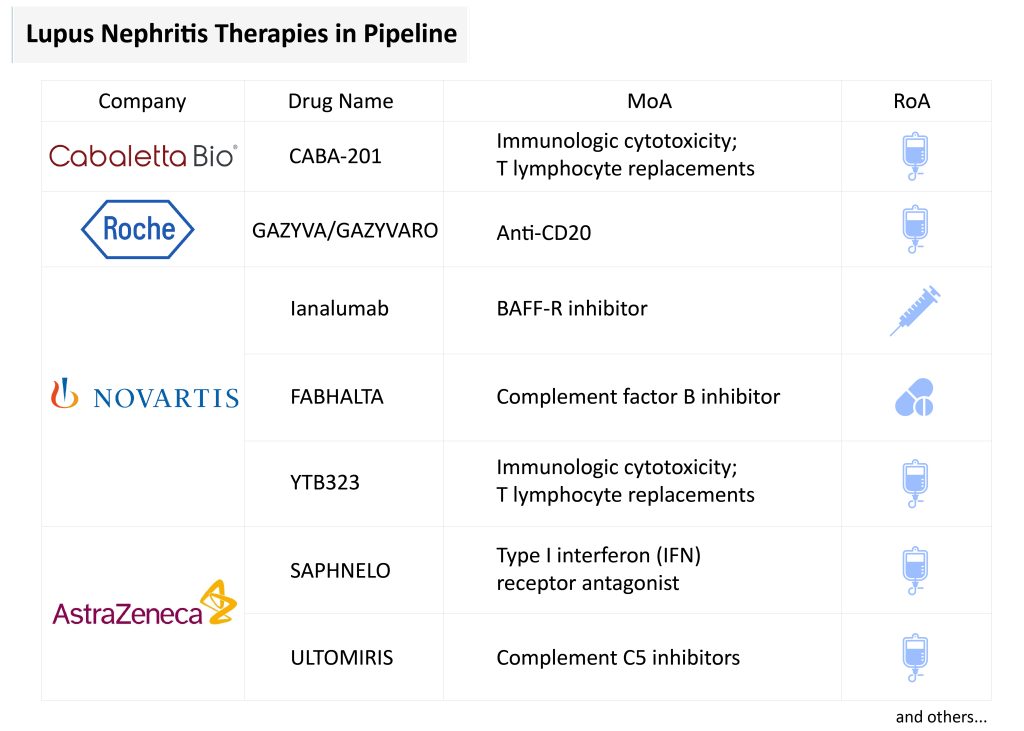
Among the most promising is obinutuzumab, a humanized type II anti-CD20 monoclonal antibody. In March 2025, the FDA accepted a supplemental Biologics License Application based on Phase III REGENCY trial data, where obinutuzumab achieved a 46.4% CRR versus 33.1% for placebo. Unlike earlier anti-CD20 agents like rituximab, it offers enhanced B-cell depletion and is the first in its class to show efficacy in a randomized Phase III lupus nephritis trial. If approved, it will be the first CD20-directed therapy specifically indicated for lupus nephritis, with projected sales of USD 400 million by 2034.
Ianalumab, a dual-action anti-BAFF-R monoclonal antibody developed by Novartis, combines B-cell depletion via ADCC with inhibition of BAFF signaling. Encouraging Phase II results in Sjögren’s syndrome suggest potential superiority over existing B-cell-targeting agents, and Phase III trials in lupus nephritis are ongoing, with regulatory submissions expected around 2028.
Cell-based lupus nephritis therapies are also making strides. Resecabtagene autoleucel (CABA-201) and YTB323 are CD19-directed CAR-T cell therapies designed to reset immune tolerance by targeting autoreactive B-cells. Early data from the RESET trial show sustained DORIS remission and complete renal response in lupus nephritis patients, with favorable safety profiles and potential for durable, one-time treatments. YTB323 leverages Novartis’ T-Charge platform and is currently in Phase II development, with lupus nephritis-specific submission targeted post-2027.
FABHALTA (iptacopan), an oral factor B inhibitor, is also under investigation for lupus nephritis, supported by its efficacy in complement-driven kidney diseases such as IgAN, PNH, and C3G. Although lupus nephritis-specific data are pending, its strong mechanistic rationale and commercial traction—USD 129 million in 2024 sales—underscore its potential role in proliferative lupus nephritis.
ULTOMIRIS (ravulizumab), AstraZeneca’s long-acting complement C5 inhibitor, is being studied in Phase II trials for Class III/IV lupus nephritis. While direct efficacy data in lupus nephritis are lacking, ULTOMIRIS’s success in complement-mediated diseases like aHUS, PNH, and NMOSD—with global sales of USD 3.9 billion in 2024—supports its candidacy for lupus nephritis through mechanistic extrapolation.
SAPHNELO (anifrolumab), a type I IFN receptor antagonist, is progressing through Phase III lupus nephritis trials. Approved for SLE in over 65 countries, SAPHNELO has shown favorable steroid-sparing effects and sustained remission. However, its Q4W IV dosing and lack of a subcutaneous option are drawbacks compared to BENLYSTA, and obinutuzumab’s superior CRR in Phase II trials poses a competitive threat.
Despite promising developments, the lupus nephritis treatment landscape has also seen setbacks. Vemircopan (ALXN2050), a complement pathway inhibitor, had its Phase II program terminated in January 2025 due to safety and efficacy concerns. Similarly, Kezar Life Sciences halted the PALIZADE trial of zetomipzomib (KZR-616) following an FDA clinical hold after four fatal SAEs. While the drug showed a 42% CRR and favorable biomarker shifts, safety concerns necessitated a redirection of efforts toward other indications. These discontinuations underscore the complexity of lupus nephritis drug development and the need for a careful balance between innovation, efficacy, and safety.
The therapeutic landscape of lupus nephritis is undergoing a profound transformation, marked by a shift from broad immunosuppression to precision-targeted interventions. Recent approvals and a robust pipeline reflect both the progress and challenges inherent in developing effective, safe, and durable treatments. While several promising lupus nephritis therapies are on the horizon—including anti-CD20 antibodies, complement inhibitors, IFN-targeting agents, and CAR-T cell therapies—long-term success will depend on overcoming safety concerns, improving response durability, and ensuring access to care. As our understanding of lupus nephritis pathophysiology deepens, future therapies will likely be increasingly tailored, offering new hope to patients facing this complex and life-threatening disease.

Downloads
Article in PDF
Recent Articles
- Aurinia’s Lupkynis for Lupus; FDA Fast Track Designation for Toripalimab; Wren Therapeutics...
- Daiichi Sankyo’s Intravenous Iron Replacement Therapy; ANeuroTech’s Adjunctive Anti-depression Dr...
- Lupus Nephritis: A SILENT but SEVERE Systemic Lupus Erythematosus Manifestation
- Roche’s Gazyva receives FDA Breakthrough designation for Lupus nephritis
- Bayer’s New Cardiology Drug Acoramidis; Two Datopotamab Deruxtecan Applications Validated in the ...