Upcoming Contenders in Dry Eye Disease Drug Space: 5 Therapies to Watch Post-TRYPTYR Approval
Jun 06, 2025
The recent approval of the dry eye disease drug TRYPTYR has heightened competition among pharmaceutical companies. Six years after spinning off from Novartis and going public, eye care company Alcon has secured its first FDA approval for a prescription medication targeting dry eye disease.
The dry eye disease drug, TRYPTYR, is a new TRPM8 receptor agonist formulated to boost tear production and is used as a single drop twice a day. The FDA’s approval was supported by results from two Phase III clinical trials in dry eye disease, which included over 930 patients with a history of the condition and were evenly randomized to receive either TRYPTYR or a placebo. In the COMET-2 and COMET-3 trials, patients treated with TRYPTYR were up to four times more likely to experience at least a 10mm increase in natural tear production by Day 14 compared to those given the vehicle—42.6% versus 8.2% in COMET-2, and 53.2% versus 14.4% in COMET-3 (both with p-values below 0.0001). These notable improvements persisted through Day 90, with TRYPTYR demonstrating a statistically significant rise in tear production as early as the first day of treatment.
The approval has triggered a race among pharma companies to introduce their assets to the market swiftly. The dry eye disease drug pipeline is rapidly advancing, featuring a wide array of treatments that offer novel mechanisms of action, convenient administration, and improved safety and efficacy profiles. Multiple pharmaceutical companies are actively developing their leading candidates across various clinical stages to transform the treatment landscape for dry eye disease.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Leo Pharma’s Hand Eczema Clinical Trial Updates; GSK’s PD-1 inhibitor Jemperli Approval; Ro...
- Amgen’s UPLIZNA Wins First FDA Nod for IgG4-Related Disease; Aldeyra Gets FDA CRL for Reproxalap ...
- Aktis’s Novel Targeted Alpha Radiopharmaceuticals; Koye Partners with Sonde Health; Novartis to S...
- Cerner, Scanwell Health and Personal Genome Diagnostics Acquisition; Zynex’s Fluid Monitori...
- How the Amniotic Membrane Market is Transforming Regenerative Healthcare Worldwide
Notable pipeline dry eye disease drugs include PL9643 by Palatin Technologies, Reproxalap from Aldeyra Therapeutics, Tivanisiran (SYL1001) by Sylentis/PharmaMar, Tavilermide from Mimetogen, SkQ1 eye drops by Mitotech, BRM421 from BRIM Biotechnology, and RGN-259 (Tβ4) developed by ReGenTree and RegeneRx Biopharmaceuticals, among others.
Now, let’s examine these dry eye disease drugs in detail
Palatin Technologies’ PL9643
PL9643 is a synthetic pan-agonist of melanocortin receptors, developed by Palatin Technologies to treat inflammatory eye conditions such as dry eye disease. It works by modulating the melanocortin receptor system, offering a novel mechanism of action that distinguishes it from currently available therapies and could enhance treatment options for DED.
In February 2024, Palatin shared results from the pivotal Phase III MELODY-1 trial, which assessed the safety and effectiveness of PL9643 versus placebo in treating DED. By August 2024, the company announced it had received FDA feedback confirming the acceptability of the trial protocols and endpoints for evaluating both signs and symptoms of DED in ongoing Phase III studies. Topline data is anticipated in the fourth quarter of 2025.
In May 2025, Palatin Technologies, Inc. shared new findings from the Phase III MELODY-1 trial at the 2025 Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting. The updated responder analysis demonstrated PL9643’s fast onset, wide-ranging and statistically significant effectiveness, and full symptom resolution across several measures in patients with dry eye disease.
These results further enhance the clinical profile of PL9643 and underscore its potential to meet a significant unmet need by delivering symptom relief that exceeds what current treatments offer.
Aldeyra Therapeutics’s Reproxalap
Reproxalap, an investigational dry eye disease drug developed by Aldeyra Therapeutics, is currently in Phase III trials for the treatment of both dry eye disease and allergic conjunctivitis. It is a first-in-class small-molecule that modulates reactive aldehyde species (RASP), aiming to reduce inflammation linked to ocular and systemic inflammatory conditions.
In Q2 2025, Aldeyra plans to announce top-line results from its ongoing field and chamber trials for dry eye disease. If the outcomes are positive and following discussions with the FDA, the company aims to resubmit the NDA around mid-2025. Additionally, Aldeyra broadened its exclusive option agreement with AbbVie. If approved, Reproxalap could represent a meaningful new treatment option for patients with dry eye disease and allergic conjunctivitis.
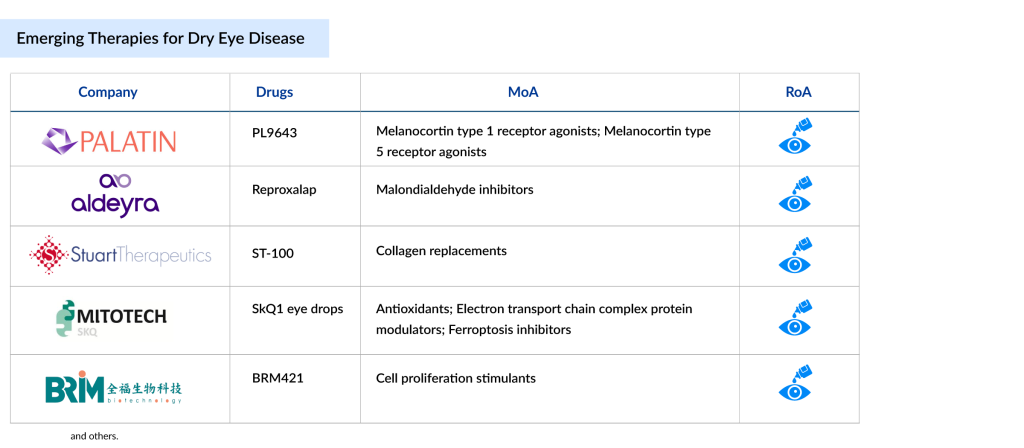
Stuart Therapeutics’ ST-100
ST-100 (vezocolmitide) is a proprietary collagen-mimicking peptide designed to repair damaged collagen in the eye, helping to restore the extracellular matrix and support normal cellular signaling. In preclinical studies, it showed benefits such as enhanced cell health and reduced inflammation and oxidative stress across epithelial, neuronal, and endothelial tissues. The therapy utilizes PolyCol technology to ease the burden of collagen remodeling in the cornea.
In January 2025, Stuart Therapeutics completed the final patient visit in its Phase III trial of ST-100 for dry eye disease. The drug met its FDA pre-approved primary endpoint, showing a reduction in overall ocular discomfort after 14 days, with effects lasting through 28 days of treatment.
Mitotech/Essex’s SkQ1 Eye Drops
SkQ1 eye drops, developed by Mitotech, are innovative mitochondria-targeted antioxidants aimed at treating dry eye disease by acting at the cellular level. They work by reducing oxidative stress and protecting tissues from degeneration, offering a novel therapeutic approach.
In Phase III clinical trials (VISTA-1 and VISTA-2), SkQ1 showed meaningful improvements in central corneal fluorescein staining and best-corrected visual acuity. Although the studies did not meet their co-primary endpoints, the drops demonstrated statistically significant benefits in key secondary measures, supporting their potential efficacy and positioning them for a possible NDA submission.
BRIM Biotechnology’s BRM421
BRM421, developed by BRIM Biotechnology, Inc., is a topical eye drop designed to treat moderate-to-severe dry eye disease. It contains a synthetic peptide inspired by pigment epithelium-derived factor (PEDF), offering both neurotrophic and anti-inflammatory effects to support corneal healing and enhance tear quality.
The key component, a PEDF-derived short peptide (PDSP), helps stimulate the regeneration and proliferation of corneal limbal stem cells, facilitating corneal repair. In March 2023, BRIM Biotechnology began its Phase III clinical trial for BRM421 by enrolling the first patient.
The anticipated launch of these emerging dry eye disease drugs is poised to significantly reshape the treatment landscape by offering novel mechanisms of action, faster onset of relief, and improved safety profiles compared to traditional therapies. These next-generation drugs address key unmet needs, such as inflammation control, tear film stability, and neurosensory abnormalities, potentially improving both patient adherence and long-term outcomes.
Their entry is likely to intensify market competition, reduce reliance on older agents like cyclosporine, and stimulate further innovation in the field. Additionally, the availability of more targeted and effective treatments may expand the pool of diagnosed and treated patients, ultimately enhancing quality of life for millions suffering from this chronic, multifactorial condition.

Downloads
Article in PDF
Recent Articles
- Amgen’s UPLIZNA Wins First FDA Nod for IgG4-Related Disease; Aldeyra Gets FDA CRL for Reproxalap ...
- FDA Expands SOLIRIS for Pediatric Myasthenia Gravis; vTv’s Cadisegliatin Program Resumes as FDA L...
- Alcon Gains CE Mark for Clareon Vivity IOL in Europe; MicroPort MedBot’s Toumai SP Robot Wins NMP...
- How the Amniotic Membrane Market is Transforming Regenerative Healthcare Worldwide
- Exploring the Growing Demand and Evolving Market Dynamics of Artificial Tears