Essential Thrombocythemia Treatment—Why the US Lags Behind Europe?
Sep 29, 2025
Table of Contents
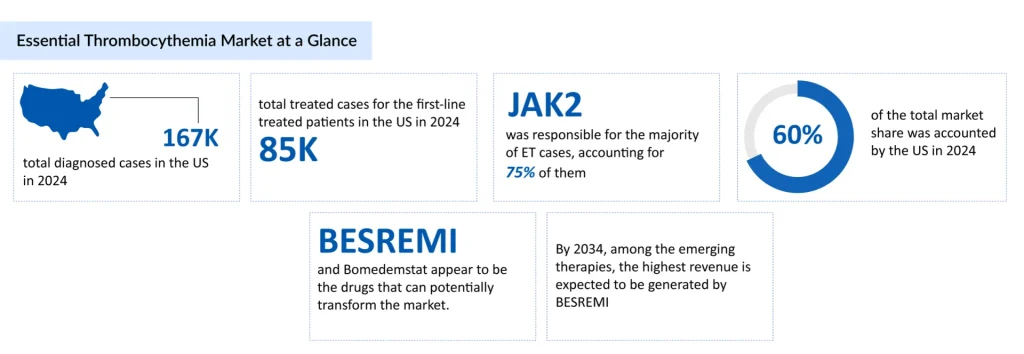
Affecting roughly 1–2 people per 100,000 each year, essential thrombocythemia is often silent, discovered during routine blood tests, yet it can quietly impact quality of life. The total number of diagnosed cases of essential thrombocythemia in the US was approximately 167,000 cases in 2024. In the United States, the highest number of age-specific cases was recorded for individuals aged 60 years and older, i.e., ~109,000 cases in 2024. Followed by the age group of 40-60 yrs and <40yrs.
While the exact cause is linked to mutations such as JAK2, CALR, or MPL, modern treatments, ranging from low-dose aspirin to targeted therapies, help manage risks and enable individuals to maintain a normal life.
Downloads
Click Here To Get the Article in PDF
Recent Articles
Key Differences in US vs. European Essential Thrombocythemia Treatment Approaches
While the overarching treatment goal for essential thrombocythemia, reducing thrombotic risk and managing symptoms, remains consistent, significant differences exist between the US and European approaches due to variations in drug approvals, clinical guidelines, and treatment philosophies.
Approved Therapies and Regulatory Landscape
Currently, PEGASYS (peginterferon alfa-2a) by pharma is the only drug specifically approved for treating essential thrombocythemia, and its authorization is limited to Europe. PEGASYS is a Type I interferon, created through pegylation, a process in which polyethylene glycol (PEG) chains are attached to interferon alfa-2a.
The essential thrombocythemia drug was previously approved by the European Commission (EC) for chronic hepatitis B treatment in adults and children aged 3 years and older, as well as chronic hepatitis C in adults and children aged 5 years and older, either in combination with other medications in adults or with ribavirin in children. In August 2024, pharmaand GmbH (pharma&) announced that the EC had granted marketing authorization for a Type II variation of PEGASYS as a monotherapy for adults with essential thrombocythemia.
In contrast, the United States has no essential thrombocythemia-specific FDA-approved therapy; treatments are primarily off-label, relying on hydroxyurea, anagrelide, or conventional interferons. This regulatory difference shapes the choice and accessibility of therapies in each region.
First-Line Treatment Preferences
European guidelines, such as those from the European LeukemiaNet (ELN), often recommend pegylated interferons as a first-line option for younger or high-risk patients due to their disease-modifying potential. Hydroxyurea remains standard for older patients or those at higher thrombotic risk. In the US, first-line therapy more commonly relies on hydroxyurea or anagrelide, with interferons used less frequently due to limited FDA indications and clinician experience.
Risk Stratification and Treatment Intensity
Risk assessment in essential thrombocythemia guides therapy in both regions, but there are nuanced differences. European guidelines often incorporate age, history of thrombosis, cardiovascular risk factors, and mutation status (JAK2, CALR, MPL) to define high-risk patients. US practice also emphasizes these factors but tends to be more conservative with aggressive therapies like interferons, often reserving them for refractory cases or clinical trial participation.
Monitoring and Long-Term Management
European clinicians typically emphasize ongoing molecular monitoring and dose adjustments, particularly with pegylated interferons, to achieve hematologic and molecular responses. In the US, monitoring practices primarily focus on blood counts and clinical events, with less emphasis on molecular remission, except in research settings.
Patient-Centric Considerations
In Europe, there is a growing trend toward individualized, disease-modifying approaches that aim to alter the course of the disease. In contrast, in the US, the focus is more on symptom control and reducing the risk of thrombosis. Patient preferences, comorbidities, and tolerability play a crucial role in shaping therapy decisions in both regions.
Why the US Lags: Systemic and Regulatory Factors
The United States lags behind other regions in certain therapeutic approvals and adoption due to a combination of systemic and regulatory factors.
Regulatory Hurdles: The FDA requires extensive post-marketing surveillance and pediatric data, even for adult essential thrombocythemia drugs, which extends review times. The EMA’s adaptive pathways allow conditional approvals based on early data, fostering innovation.
Pharmaceutical Economics: US drug prices are the world’s highest, incentivizing companies to prioritize lucrative markets but delaying generics. Europe’s price controls (e.g., via the INN system) encourage faster approvals to recoup investments.
Funding and Research Priorities: Europe’s collaborative funding (e.g., Horizon Europe grants) supports MPN research consortia, yielding breakthroughs like next-generation JAK inhibitors. US NIH funding for rare diseases, such as essential thrombocythemia, is limited ($5-10 million annually), compared to Europe’s €50 million or more through joint programs.
Healthcare Fragmentation: The US’s privatized system leads to variability in care; rural patients with essential thrombocythemia may lack access to hematologists, unlike those in Europe’s integrated networks.
Essential Thrombocythemia Therapies on the Horizon
A few emerging therapies, such as BESREMi (Ropeginterferon alfa-2b) (PharmaEssentia), Pelabresib (Novartis), Bomedemstat (MK-3543/IMG-7289) (Merck), and others, are being developed in late-stage essential thrombocythemia clinical trials.
Ropeginterferon alfa-2b is an innovative, site-specific, monopegylated, and stable IFN-α analog. Its distinct single isoform sets it apart from earlier polypegylated interferons, which relied on random pegylation methods and consequently comprised multiple isoforms, each with varying activity and stability due to different PEG conjugates.
PharmaEssentia is conducting essential thrombocythemia clinical trials to evaluate the efficacy and safety of ropeginterferon alfa-2b, while AOP Orphan frequently partners in Europe for regulatory approvals and distribution. Currently, ropeginterferon alfa-2b is FDA-approved for treating polycythemia vera, and the company plans to engage with the FDA to expand its label to include essential thrombocythemia (ET), with regulatory submission expected by the end of 2025.
Bomedemstat (MK-3543) is an experimental, small-molecule, irreversible LSD1 inhibitor under development by Merck. LSD1 plays a critical role in regulating hematopoietic stem cell proliferation and in controlling cell differentiation and maturation. Bomedemstat is being tested across multiple myeloproliferative neoplasms (MPNs), including ET, myelofibrosis (MF), and polycythemia vera (PV). The drug received Orphan Drug Designation (ODD) for ET in 2018, and in January 2020, Imago was granted Fast Track Designation (FTD) by the FDA for its use in ET.

Pelabresib is an investigational, selective small-molecule therapy designed to enhance anti-tumor activity by inhibiting bromodomain and extra-terminal (BET) proteins, potentially downregulating genes involved in hematologic malignancies. The ongoing Phase II MANIFEST trial (NCT02158858) is evaluating pelabresib in an open-label study of patients with myelofibrosis and high-risk ET.
The expected launch of potential therapies by key players in the market during the forecast period (2025–2034) is expected to create a positive impact on the market size of essential thrombocythemia in the US.
Future US Essential Thrombocythemia Treatment Market Outlook Looks Promising
The US treatment market for essential thrombocythemia appears increasingly promising, driven by a combination of heightened disease awareness, improved diagnostics, and a surge in targeted clinical programs, which are fueling growth. As per DelveInsight analysis, the US essential thrombocythemia market is expected to rise from USD 263 million in 2024 by 2034, as novel agents move through development and the adoptive off-label use of targeted drugs increases.
That upside is supported by the small current therapeutic baseline; clinicians today rely mainly on cytoreductive agents (hydroxyurea, anagrelide) and interferons, with several targeted approaches and JAK-pathway strategies being tested to address unmet needs in efficacy and tolerability, meaning any safe, disease-modifying entrant could capture meaningful share. Recent clinical readouts and ongoing trials suggest real near-term potential for new, more precise options to shift treatment patterns and expand the addressable market in the U.S. if safety and regulatory milestones are met.

Downloads
Article in PDF