Achondroplasia Treatment: Emerging Therapies, Pathway Strategies, and Market Outlook
Nov 21, 2025
Table of Contents
Achondroplasia, the most common form of dwarfism, is drawing increasing interest from pharmaceutical companies despite its rarity. Biomarin’s VOXZOGO (vosoritide; a C-type natriuretic peptide; once-daily subcutaneous injection) is the only FDA-approved therapy for achondroplasia treatment. When it comes to BioMarin’s approved therapy portfolio, VOXZOGO is the game-changer. It is also the reason why BioMarin’s revenues have increased over the years. In 2023, the VOXZOGO’s label was expanded to include children under five years old (all children with achondroplasia and open epiphyses), after it was approved in 2021 to treat achondroplasia (children ages five and older). VOXZOGO generated USD 735 million in global revenue in 2024 (24% contribution from the United States and a 76% contribution from outside of the United States), with 47 countries contributing to VOXZOGO’s global revenues. According to Biomarin, the growth in VOXZOGO’ revenue is attributed to;
Exclusivity: Expected to remain the only approved product for achondroplasia, from birth, through 2027.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- FDA Approves PENBRAYA for Most Common Serogroups Causing Meningococcal Disease; BIMZELX Approved ...
- Achondroplasia Treatment Space: Hunt for Potential Curative Therapies
- Beam’s BEAM-302 Earns FDA Orphan Drug Tag for AATD; Incannex’s IHL-42X Moves to Phase III After F...
- Novo Nordisk Seeks FDA Approval for Higher-Dose 7.2 mg WEGOVY Injection; Ascendis Pharma Granted ...
Patient Demographics: Since the 2023 label expansion, the majority of new patients are in the 0–5 age group.
Prescriber Expansion: Continued growth in the US prescriber base, especially pediatric endocrinologists.
Awareness and Referrals: Strong progress in raising awareness, driving referrals from pediatricians.
Clinical Data and Guidelines: New treatment guidelines for VOXZOGO and a growing body of clinical benefit data beyond height.
By 2027, Biomarin expects VOXZOGO to be available in more than 60 countries.
Which Therapy is Expected to Compete with Biomarin’s VOXZOGO?
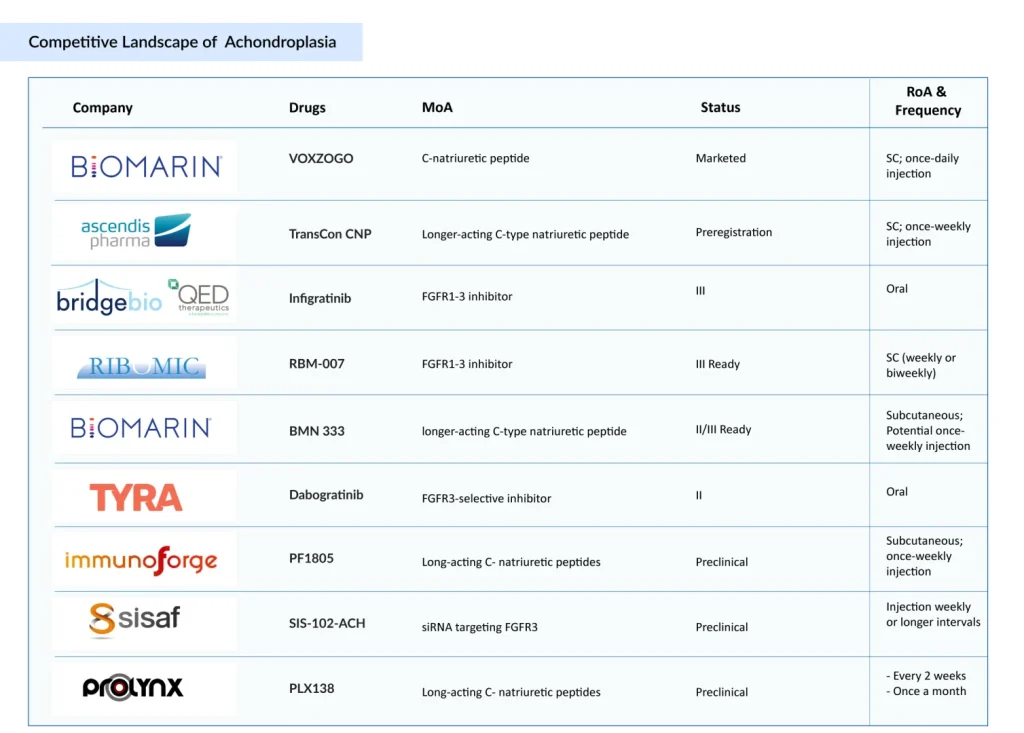
The treatment landscape of achondroplasia is evolving rapidly, with new entrants like Ascendis Pharma (TransCon CNP), BridgeBio (infigratinib), Ribomic (RBM-007) bringing competition into focus. All four of these players target either the fibroblast growth factor (FGF) signaling pathway or aim to boost C-type natriuretic peptide (CNP) activity — two distinct but interconnected therapeutic strategies addressing the core pathophysiology of achondroplasia.
BioMarin’s monopoly in the achondroplasia treatment market is expected to be challenged by once-weekly TransCon CNP (C-type natriuretic peptide) from Ascendis Pharma. When Ascendis Pharma announced TransCon CNP results, BioMarin’s stock fell 17%. Ascendis Pharma’s TransCon CNP has a PDUFA date of November 30, 2025 (filed an NDA with the FDA on March 31, 2025). Despite the drawbacks of cross-trial comparisons, the TransCon CNP profile seems to be competitive in terms of efficacy, safety, and convenience, and we believe TransCon CNP could become a significant product for Ascendis:
- Demonstrated statistically significant height gains over placebo in pivotal trials.
- The once-weekly dosing is a strong differentiator.
- Low rate of mild side effects and good tolerability.
- Under FDA Priority Review, with a decision expected in November 2025.
Even though TransCon CNP has a better profile and results look competitive with BioMarin’s VOXZOGO, it is important to note that it has not yet received FDA approval or entered the market. There could be some challenges, such as:
- Biomarin, seeking to block competition, filed an action with the International Trade Commission (ITC) on April 1, 2025 (the day after Ascendis submitted its NDA to the FDA), alleging that Ascendis’ imported TransCon CNP from Europe infringes Biomarin’s RE267 patent. Biomarin’s move targets Ascendis’ drug development, as importing a patent-infringing drug can create liability even before sales begin. Essentially, Biomarin is attempting to use the ITC case to stifle Ascendis’ development of a competing therapy.
- The geographic footprint of VOXZOGO spans around 47 countries and is expected to expand to more than 60 by 2027. VOXZOGO’s 75% revenue base is outside the US. Competitors are going to maintain a similar market presence.
- The majority of children in the United States are actually diagnosed prenatally, and often, the decision to treat is made prenatally. Biomarin is the only one that is indicated in infants.
Why are Companies Extensively Targeting FGFR3 and CNP Pathways?
The marketed and emerging landscape of achondroplasia indicates that researchers are extensively investigating the FGFR3 and CNP pathways. The rationale for exploiting these pathways is that FGFR3 and CNP pathways are tightly linked to the molecular root of achondroplasia, making them logical and potentially effective targets. Achondroplasia is caused by gain-of-function mutations in the FGFR3 gene, leading to excessive activation of the FGFR3 receptor. Two mutations, G1138A and G1138C, in exon 10 of the FGFR3 gene result in a specific amino acid substitution (G380R) and account for more than 97% of all reported cases.
This overactivation inhibits chondrocyte proliferation and differentiation, effectively stalling bone growth at the growth plate. The FGFR3 pathway activates a cascade of intracellular signals, particularly through the RAS-ERK (MAPK) pathway, which is directly responsible for many of the skeletal abnormalities seen in achondroplasia. To counter this, two main treatment strategies have emerged:
FGF Pathway Inhibition: These therapies block FGFR3 directly or inhibit its downstream signals, aiming to dial down the overactive pathway.
CNP Supplementation: These therapies enhance CNP signaling, which naturally counteracts FGFR3 activity by activating the NPR-B receptor and increasing cGMP production, thereby dampening MAPK pathway activation.
Blocking FGFR3 can have broader and more potent effects, as it halts signaling at its source. CNP analogs, on the other hand, modulate the pathway further downstream, offering a more physiologically balanced approach that complements the body’s natural regulatory systems.
| Challenges and Limitations of Each Strategy: FGFR3 Inhibition and CNP-Based Therapies | ||
| Parameter | FGFR3 inhibitors | CNP-Based Therapies |
| Specificityand half-life | FGFRs are involved in many biological systems, so there is a potential risk of off-target effects | – CNP has a very short half-life, but next-gen versions aim for weekly or monthly dosing- NPR-B/cGMP pathway is more selective to cartilage, but still not entirely bone-specific |
| Safety profile | – Long-term safety in children is still under evaluation- Risk of interfering with brain, tooth, or skull development | Risk of hypotension at higher doses |
| Dosing | Oral, but systemic exposure increases toxicity risks | Daily or weekly injections can be burdensome |
The Future of Achondroplasia Drug Development: Is It Time to Look Beyond FGFR3 and CNP?
Therapeutic approaches for achondroplasia directly or indirectly target the overactive FGFR3. The achondroplasia drug pipeline has largely centered on targeting FGFR3 signaling (the root genetic cause) and CNP pathways (to promote bone growth). However, recent competition and patent disputes like Biomarin’s patent action against Ascendis highlight the challenges of relying on these same pathways. Innovation beyond FGFR3 and CNP could open new avenues for safer and more effective treatments for patients with achondroplasia. To date, no alternative therapies outside the FGFR3/CNP signaling axis have succeeded in clinical development. Some investigational approaches explored include:
- Teriparatide: Promoted bone growth in animal models, but has not advanced clinically.
- Statins: Showed some promise in mice, but failed to replicate in chondrocytes.
- Growth Hormone (GH): Approved in Japan, but with modest and variable efficacy.
- Osteocrin: Explored in animal models, but no data in achondroplasia models.
Barriers to Innovation: What’s Holding Back Progress?
BioMarin’s VOXZOGO obtained regulatory approval in 2021, and to date, no other treatments have been approved. This suggests that developing therapies for achondroplasia is not without hurdles:
Biological complexity: FGFR3 is a crucial developmental pathway; modulating it is risky.
Trial design issues: Small patient pools, and long-term follow-up needs.
Safety: Children may be on treatment for years, so proving long-term safety is critical. For example;
Anatomical outcomes: Improving height is just one piece; preventing complications like spinal stenosis or hydrocephalus remains a challenge.
Small Patient Population but Large Market Potential: Why Achondroplasia Is Attracting Pharma Investment?
In the 7MM (in 2024), around 28,500 diagnosed prevalent cases of achondroplasia were estimated. Despite the small patient population, the achondroplasia market is commercially attractive for several reasons:
- High-unmet need: Children face serious medical and functional complications. Early intervention is particularly important because, left untreated, the skeletal disease leads to complications such as obesity and posture problems.
- Established precedent and billion-dollar market opportunity: VOXZOGO has paved a clear regulatory path for future drugs. VOXZOGO’s global revenue guidance for 2025 is between USD 900-930 million. This suggests that demand has been growing rapidly.
- Premium pricing: Orphan drugs often command high prices, supporting profitability even with limited users. VOXZOGO is launched at a hefty cost of USD 320,000 per year.
- Orphan drug incentives: Orphan drug status offers regulatory incentives, market exclusivity, and premium pricing that can make drug development financially attractive.
The market size of achondroplasia (in 2024) is estimated around USD 300 million, which is expected to grow to approximately USD 1.5 billion by 2034, driven by more diagnoses, earlier treatment initiation, longer treatment duration, and the entry of new and better therapies.
Conclusion
The future of achondroplasia treatment is increasingly promising, driven by scientific clarity on its genetic roots and strong momentum in drug development. Ascendis’ likely approval of TransCon CNP for achondroplasia poses a major threat to BioMarin’s top-selling drug; however, VOXZOGO and BMN 333 together could successfully counteract this. To date, the FGFR3/CNP axis remains the most viable and evidence-supported target for drug development. Up until now, the major focus of the achondroplasia pipeline has been on small molecules, but treatment dynamics is slowly moving towards siRNA (small interfering RNA). While ImmunoForge and ProLynx are developing fusion peptides and extended-release CNP formulations to improve durability, companies such as SiSaf are investigating siRNA-based FGFR3 suppression.

Downloads
Article in PDF
Recent Articles
- Beam’s BEAM-302 Earns FDA Orphan Drug Tag for AATD; Incannex’s IHL-42X Moves to Phase III After F...
- FDA Approves PENBRAYA for Most Common Serogroups Causing Meningococcal Disease; BIMZELX Approved ...
- Novo Nordisk Seeks FDA Approval for Higher-Dose 7.2 mg WEGOVY Injection; Ascendis Pharma Granted ...
- Achondroplasia Treatment Space: Hunt for Potential Curative Therapies