The Evolution of Vascular Access Devices in Modern Medicine
Jan 21, 2026
Table of Contents
In the intricate world of modern clinical practice, the ability to safely and reliably access a patient’s circulatory system is perhaps the most fundamental requirement of acute and chronic care. Vascular Access Devices (VADs), ranging from simple peripheral catheters to complex implanted ports, serve as the “highways” for life-saving medications, nutritional support, and diagnostic monitoring.
As we move toward a future of precision medicine and “hospital-at-home” models, the technology behind these devices is undergoing a radical transformation. This article explores the historical foundations, clinical evolution, and the burgeoning market landscape of vascular access, where advanced materials and artificial intelligence are redefining patient safety.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- FDA Grants 510(k) Clearance to Neonav® ECG Tip Location System; ClearPoint Neuro Secures EU MDR C...
- Parenteral Nutrition: A High-Growth Lifeline in Clinical Nutrition
- Collagen Matrix FDA 510(k) approval for Fibrillar Collagen Wound Dressing; Roche’s cancer diagnos...
- QuantuMDx & Menarini’s Agreement; TruClear System Launched by Medtronic in India; FDA 510(k)...
Historical Foundations: From Glass to Silicone
The journey of vascular access began with rudimentary and often dangerous experiments. In the mid-19th century, early attempts at intravenous therapy utilized glass cannulae and metal trocars. These materials were inherently rigid and prone to causing significant vessel trauma, thrombosis, and infection. The primary limitation was “biocompatibility”, a concept that early pioneers like William Harvey and Richard Lower understood only in principle.
The 20th century marked a pivot toward safer materials. The shift from glass to silicone and, eventually, to advanced polymers like polyurethane and Teflon revolutionized the field. Silicone, introduced in the mid-1960s, offered a soft, flexible alternative that could dwell within a vessel for extended periods without triggering the body’s defensive inflammatory response. This transition allowed clinicians to move from “snapshot” infusions to sustained therapeutic windows, laying the groundwork for modern oncology and intensive care.
The Rise of Sophisticated Venous Access: Tunneled and Peripherally Inserted Catheters
As the need for long-term therapy grew, the medical community required devices that could stay in place for months or even years. This led to two distinct but complementary technological paths:
Central Venous Access and Tunneled Catheters
In the 1970s and 80s, the development of tunneled central venous catheters (tCVCs), such as the Hickman and Broviac lines, provided a solution for patients requiring high-volume infusions or frequent blood sampling. By “tunneling” the catheter under the skin before it enters the vein, clinicians created a physical barrier against bacteria, significantly reducing the risk of infection. These devices became the gold standard for bone marrow transplants and long-term parenteral nutrition.
The Peripherally Inserted Central Catheter (PICC) Era
The introduction of the Peripherally Inserted Central Catheter (PICC) represented a paradigm shift in nursing and interventional radiology. PICC lines provide central venous access via a peripheral vein (usually in the arm), avoiding the high-risk “blind” sticks into the neck or chest. This era democratized central access, moving it from the operating room to the bedside and significantly reducing procedural complications such as pneumothorax.
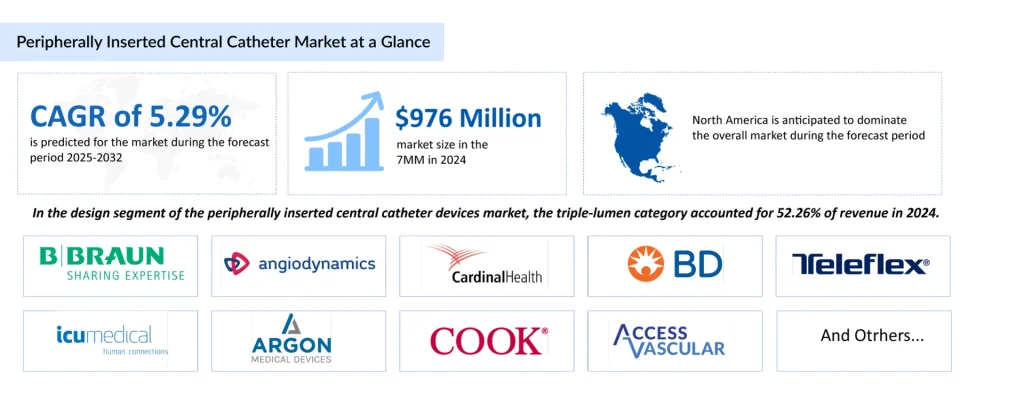
Addressing the Infection Challenge: A Clinical Imperative
Despite technological gains, Catheter-Related Bloodstream Infections (CRBSIs) remain the “Achilles’ heel” of vascular access. These infections extend hospital stays, increase healthcare costs by billions annually, and, most importantly, carry a mortality rate of up to 25%.
In response, the industry has undergone a paradigm shift, moving beyond simple plastic tubes toward “active” device designs and integrated safety ecosystems. Modern clinical protocols now prioritize a holistic “bundle” of care designed to eliminate points of failure. This includes the rigorous application of the Aseptic Non-Touch Technique (ANTT) to standardize insertion and maintenance, as well as the universal adoption of needle-free connectors and closed systems that serve as critical barriers against ambient air and bacterial ingress. Furthermore, there is a decisive shift toward advanced securement methods, with traditional sutures replaced by specialized adhesive devices. These innovations minimize catheter “pistoning”, the back-and-forth movement within the vein that serves as a primary driver of internal vessel damage and the subsequent migration of pathogens into the bloodstream.
The Intelligent Pipeline: AI and Machine Learning in Vascular Access
The future of vascular access is “smart,” shifting from manual expertise to data-driven precision. Artificial Intelligence (AI) and Machine Learning (ML) are being integrated across the entire lifecycle of a device, from initial placement to long-term monitoring.
AI-Guided Placement (The “AI-GUIDE” Era)
One of the most transformative advancements in the field is the development of handheld robotic devices that use Convolutional Neural Networks (CNNs) to interpret ultrasound images in real time, effectively bridging the skill gap for non-expert clinicians. This technology democratizes high-precision vascular access by providing an automated layer of “intelligence” that identifies and labels critical anatomical structures, such as veins and arteries, while filtering out visual noise common to raw ultrasound feeds.
By employing sophisticated deep-learning algorithms, these systems can instantly analyze thousands of data points to select the target vessel with the most favorable diameter and depth for cannulation. Furthermore, they address the high-stress Difficult Intravenous Access (DIVA) dilemma through integrated robotic needle guidance. This ensures the needle trajectory is perfectly aligned and the vessel is successfully punctured on the first attempt, significantly reducing the trauma, time, and patient discomfort historically associated with repeated “blind” insertion attempts.
Predictive Monitoring and Early Warning Systems
AI is now moving beyond reactive care to predictive intelligence, utilizing sophisticated models to anticipate complications before they escalate into clinical emergencies. In the specialized field of hemodialysis, advanced AI models are being deployed to mitigate the high risk of vascular access failure, with some systems demonstrating the ability to predict up to 75% of thrombosis cases a full week in advance. These models leverage a multi-faceted approach, analyzing longitudinal dialysis treatment data, laboratory results, and patient demographics to provide nephrologists with an early-warning window for preemptive intervention.
Complementing these clinical insights, the emergence of Artificial Intelligence of Things (AIoT) devices is revolutionizing home care for hemodialysis patients. For example, wearable SmartPatch systems and specialized home-monitoring devices now allow for continuous fistula surveillance outside the hospital. These devices utilize embedded microphones to capture vascular sound signals, which are then analyzed by Convolutional Neural Networks (CNNs) to identify “slight blockages” with an accuracy rate exceeding 93%. When a potential dysfunction is detected, the system immediately transmits an actionable alert to the hospital information system, ensuring that patients living in remote areas maintain a “digital bridge” to life-saving specialist care.
Computer Vision and Aneurysm Staging
Mobile applications are currently in development that use smartphone images to identify and classify vascular access aneurysms. By simply taking a photo, a caregiver can use AI to determine the severity of a bulge and automatically flag high-risk cases for immediate clinical intervention.
Emerging Technologies and Future Innovations
We are currently entering the “Smart Era” of vascular access, where the device is no longer a passive conduit but an active participant in the healing process.
Antimicrobial Coatings and Biomimetic Design
The latest generation of catheters features antimicrobial coatings (such as chlorhexidine or silver) and antithrombogenic surfaces. These “biomimetic” designs mimic the natural lining of blood vessels (the endothelium), thereby preventing the formation of fibrin sheaths and bacterial biofilms. This reduces the two greatest risks of long-term access: infection and occlusion.
Artificial Intelligence and Real-Time Monitoring
AI is revolutionizing how we place and monitor these devices. AI-guided ultrasound systems now assist clinicians in identifying the optimal vein and needle trajectory, significantly increasing first-pass success rates even in “difficult intravenous access” (DIVA) patients. Furthermore, Smart VADs equipped with internal sensors are being developed to monitor blood flow, pressure, and local pH levels, alerting the care team to the earliest signs of a clot or infection before clinical symptoms appear.
Wireless Ultrasound and Point-of-Care Innovation
The shift toward Wireless Ultrasound and Point-of-Care (POCUS) technologies has made bedside confirmation of catheter tip placement a reality. By using wireless probes connected to tablets or smartphones, clinicians can ensure the catheter tip resides in the lower third of the Superior Vena Cava (the “sweet spot” for central access) without requiring a confirmatory X-ray, thereby reducing radiation exposure and speeding up treatment.

Conclusion: The Path Forward
The evolution of vascular access devices is a testament to the power of iterative innovation. From the brittle glass tubes of the past to the AI-enhanced, antimicrobial-coated polymers of today, each step has been driven by a singular goal: Patient Safety.
The global vascular access device market is on a high-growth trajectory, driven by the rising prevalence of chronic conditions like cancer and kidney failure. According to recent DelveInsight data, the market is poised to reach USD 8.4 billion by 2032, with a robust CAGR of approximately 6%.
Additionally, the market is witnessing the surge due to the active participation of leading companies such as Cook, BD, Teleflex Incorporated, Medtronic, Asahi Kasei Corporation, B. Braun SE, PRODiMED, AMECATH, Terumo Corporation, AngioDynamics, ICU Medical, Inc., Nipro Medical Corporation, Medline Industries, LP, Access Vascular, Inc., Vygon Group, Cordis, Merit Medical Systems, Cardinal Health, Fresenius SE & Co. KGaA, Argon Medical Devices, and others.
In summary, as the market continues its steady climb, the focus will inevitably shift toward predictive maintenance and fully autonomous placement. In this future, vascular access will not just be a necessary procedural step; it will be a sophisticated, data-rich component of the patient’s personalized care plan.

Downloads
Article in PDF
Recent Articles
- Collagen Matrix FDA 510(k) approval for Fibrillar Collagen Wound Dressing; Roche’s cancer diagnos...
- Parenteral Nutrition: A High-Growth Lifeline in Clinical Nutrition
- QuantuMDx & Menarini’s Agreement; TruClear System Launched by Medtronic in India; FDA 510(k)...
- FDA Grants 510(k) Clearance to Neonav® ECG Tip Location System; ClearPoint Neuro Secures EU MDR C...



