Artificial Intelligence in Clinical Trials: Transforming Drug Development Efficiency and Precision
Oct 08, 2025
Table of Contents
The integration of Artificial Intelligence in clinical trials marks one of the most transformative shifts in modern drug development. As the pharmaceutical industry grapples with rising R&D costs, long trial timelines, and high attrition rates, AI in clinical trials has emerged as a catalyst for optimizing design, accelerating patient recruitment, enhancing monitoring, and improving overall trial success rates.
By harnessing advanced algorithms, predictive analytics, and real-world data, AI clinical trial technologies are reshaping the traditional trial paradigm, making studies more adaptive, data-driven, and patient-centric. Major pharmaceutical companies and CROs are increasingly embedding AI throughout the clinical trial lifecycle, from protocol design to post-marketing surveillance.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- BioGX’s ‘pixl’ Portable qPCR Platform; Stryker’s Citrefix Suture Anchor System; Boston Scientific...
- Solis Mammography’s Acquisition of MUSIC Imaging Center; Nikon’s SI-PH Phase Condenser Accessory ...
- Mindray’s TE Air Handheld Ultrasound System; Getinge’s iCast™ Covered Stent System; Clinic...
- Sleep Aids Market: Examining Cutting-Edge Technologies and Major Breakthroughs
- Medtronic Announced World’s First Approval for MiniMed 780G System; CE Mark for Medtronic’s...
This article examines how AI in clinical trials is revolutionizing research efficiency, regulatory compliance, and patient outcomes, while also uncovering the latest innovations, collaborations, and ethical challenges that define this rapidly evolving field.
Want to see how AI is transforming diagnostics across healthcare? Read here: AI-Driven Diagnostics
The Rise of AI in Clinical Research
In the past decade, Artificial Intelligence has evolved from a promising analytical tool to a fundamental pillar in reshaping how clinical trials are conceived, conducted, and evaluated. The clinical research ecosystem, historically reliant on manual processes, fragmented datasets, and linear study designs, is now entering a new era of digital transformation powered by AI-driven intelligence.
Several key factors have driven the rise of AI in clinical research, the explosion of biomedical data, advancements in machine learning (ML) algorithms, and the increasing demand for precision and efficiency in drug development. According to industry estimates, the integration of AI in clinical development could reduce trial costs by up to 15–20% and shorten timelines by nearly a year, significantly accelerating the time-to-market for life-saving therapies.
Pharmaceutical giants such as Pfizer, Novartis, and Roche are leading the adoption wave, using AI to predict patient responses, automate trial design, and identify novel biomarkers. At the same time, technology innovators like Deep6 AI, Unlearn.AI, and QuantHealth are leveraging real-world data and digital twins to model virtual control arms and optimize trial protocols before the first patient is enrolled.
This convergence of AI and clinical research is not merely enhancing operational efficiency, it’s redefining scientific rigor and inclusivity. With predictive recruitment, decentralized trial models, and real-time monitoring, AI enables studies to reach diverse patient populations while improving adherence and data accuracy.
As AI continues to mature, its role is expanding beyond optimization into innovation, ushering in the age of adaptive, continuous, and intelligent clinical development.

Current Challenges in Traditional Clinical Trials
Despite being the gold standard for assessing therapeutic safety and efficacy, traditional clinical trials face persistent challenges that limit efficiency, diversity, and success rates. These obstacles have long hindered the speed and scalability of drug development, making innovation through AI in clinical trials increasingly essential.
One of the most pressing issues is patient recruitment and retention. Nearly 80% of clinical trials fail to meet enrollment deadlines, and around 30% of participants drop out before completion. This leads to extended timelines, increased costs, and sometimes incomplete datasets. Recruitment challenges are often compounded by restrictive inclusion criteria and geographic limitations that prevent the representation of diverse populations.
Another major hurdle lies in data fragmentation and manual data handling. Traditional trials generate massive amounts of clinical, imaging, genomic, and patient-reported data, often stored in disconnected systems. The reliance on paper-based or semi-digital documentation increases the risk of human error, delays, and inconsistent reporting.
High costs and long timelines also remain key pain points. It can take over a decade and billions of dollars to bring a new drug from discovery to market, with clinical testing accounting for a significant portion of the expenditure. Failures in later stages, often due to unexpected toxicity or inefficacy, underscore the need for better predictive tools and adaptive trial models.
Lastly, limited real-world applicability continues to affect outcomes. Many trials are conducted in tightly controlled environments that don’t accurately reflect the diversity of real-life patients or the complexity of diseases. This gap between trial data and clinical practice limits translational success and post-approval performance.
These systemic issues render traditional trial models unsustainable in the long term, creating a compelling case for integrating AI-driven technologies that can streamline processes, predict risks, and enhance both scientific and operational outcomes.
How AI is Transforming the Clinical Trial Landscape
The integration of Artificial Intelligence in clinical trials is fundamentally reshaping how studies are designed, executed, and analyzed. From predicting patient eligibility to automating data monitoring, AI-driven systems are addressing inefficiencies that have long plagued traditional trials.
One of the most transformative applications lies in AI-enabled patient recruitment and matching. Algorithms analyze vast datasets, such as electronic health records (EHRs), genomics, and wearable device data, to identify patients who fit precise trial criteria. For instance, Deep6 AI’s platform reportedly accelerates patient recruitment by up to 10 times faster compared to manual screening. Similarly, Pfizer and IBM Watson collaborated to utilize AI for matching oncology trials, streamlining eligibility identification across multiple cancer subtypes.
AI is also revolutionizing trial design and protocol optimization. Predictive modeling can simulate trial outcomes under various conditions, enabling sponsors to identify the most efficient and ethical designs before enrolling a single patient. AstraZeneca and GSK, for example, have utilized machine learning (ML) tools to model patient responses and refine study endpoints, thereby reducing unnecessary complexity and dropout rates.
In the realm of data collection and monitoring, AI automates the aggregation of multi-modal data from EHRs, imaging, lab reports, and wearable devices. Natural language processing (NLP) converts unstructured clinical notes into analyzable datasets, thereby enhancing real-time safety monitoring and facilitating the detection of adverse events. According to Deloitte, this has the potential to cut data verification time by 50–60%.
Decentralized clinical trials (DCTs), powered by AI and digital health platforms, have emerged as a significant trend post-pandemic. They enable remote monitoring, telemedicine-based assessments, and digital consent, increasing patient accessibility and retention. Companies like Medable and Science 37 utilize AI-driven analytics to personalize engagement and monitor adherence, resulting in a more than 30% increase in patient compliance rates in remote studies.
Finally, AI is enhancing trial outcome prediction and decision-making. By integrating biomarkers, imaging, and clinical data, AI models can predict therapeutic response or disease progression early in the trial phase, allowing for faster go/no-go decisions and adaptive protocol adjustments.
In short, AI is transforming clinical trials from reactive, time-intensive processes into proactive, data-driven systems that prioritize speed, accuracy, and patient-centricity.
Key Applications of AI Across Clinical Trial Phases
Artificial Intelligence is now being applied throughout the entire clinical trial lifecycle to enhance efficiency, reduce costs, and improve outcomes. From initial design to post-marketing analysis, AI tools are enabling more intelligent decision-making and more patient-centric studies.
Trial Design and Protocol Optimization
AI algorithms can simulate multiple trial designs to predict which approach is most likely to succeed. This allows sponsors to optimize inclusion and exclusion criteria, identify potential bottlenecks, and select primary and secondary endpoints with greater confidence. Companies like GSK and AstraZeneca have reported reductions in protocol amendments and faster trial startup times using predictive modeling tools.
Site Selection and Feasibility
Machine learning models analyze historical trial performance, patient demographics, and site capabilities to recommend optimal locations. This improves enrollment rates and reduces delays. For example, AI-driven site selection has helped some oncology trials achieve 90% of their enrollment targets within planned timelines.
Patient Recruitment and Matching
AI platforms analyze electronic health records, genomics, and wearable data to identify suitable participants. Platforms such as Deep6 AI can match patients to trials up to ten times faster than manual screening, improving diversity and enrollment speed.
Real-Time Monitoring and Risk Prediction
AI systems continuously monitor patient data for adverse events or protocol deviations. Predictive analytics can forecast risk trends and alert investigators before issues escalate. This proactive monitoring has reduced trial monitoring costs by an estimated 30-40% in several cardiovascular and oncology studies.
Data Cleaning and Quality Control
Natural language processing and automated anomaly detection tools convert unstructured clinical notes into structured data, flag inconsistencies, and ensure regulatory compliance. Companies like Octozi have demonstrated that automated AI-driven cleaning can cut data validation time in half while improving accuracy.
Regulatory Documentation and Reporting
AI is being used to streamline the preparation of regulatory submissions, automate report generation, and maintain audit trails. This reduces human error and accelerates review timelines for both the FDA and EMA.
Across these phases, AI is moving trials toward a predictive, adaptive, and patient-focused model, ensuring that clinical development becomes faster, safer, and more reliable.
Curious about AI’s impact on pathology and imaging? Read “The Rise of AI-Enabled Digital Pathology.”
Notable Case Studies and Breakthroughs in AI-Driven Trials
The adoption of Artificial Intelligence in clinical trials is no longer theoretical. Several real-world examples demonstrate measurable improvements in trial efficiency, patient engagement, and outcome prediction.
Deep6 AI for Oncology Trials
Deep6 AI leverages machine learning to analyze electronic health records and match patients to relevant oncology trials. In one study, patient recruitment was accelerated by up to ten times compared to conventional manual methods. This technology also increased diversity in trial populations by identifying underrepresented patient groups.
Octozi for Data Cleaning and Quality Assurance
Octozi uses AI algorithms to detect inconsistencies, clean unstructured data, and flag potential errors before submission. Trials employing Octozi reported a 50% reduction in data validation time, improving both regulatory compliance and overall trial efficiency.
Unlearn.AI for Virtual Control Arms
Unlearn.AI builds digital twins of patients using historical trial data to create virtual control arms. In recent Phase II oncology trials, this approach reduced the number of participants required in control groups while maintaining statistical rigor. It also shortened study timelines and minimized patients’ exposure to placebos.
Medable and Science 37 in Decentralized Trials
Both Medable and Science 37 combine AI analytics with telemedicine to conduct decentralized trials. AI tracks adherence, flags anomalies in real time, and personalizes patient engagement. These strategies have increased patient retention rates by over 30% in remote cardiovascular and neurological studies.
Generative AI for Protocol Drafting
Emerging generative AI platforms are now assisting in drafting clinical protocols and regulatory documents. These tools reduce repetitive work, improve accuracy, and allow clinical teams to focus on study design and patient outcomes.
These case studies demonstrate that AI is not just a supportive tool but a strategic enabler capable of transforming how trials are conducted. With faster recruitment, improved data quality, and predictive analytics, AI-driven clinical trials are setting new standards for efficiency and patient-centricity.
Explore how AI is improving patient care in real-time settings: Read here: The Rise of AI-Powered Point-of-Care Diagnostics
Regulatory and Ethical Considerations for AI in Clinical Trials
While Artificial Intelligence offers significant advantages, its integration in clinical trials raises important regulatory and ethical questions. Ensuring transparency, patient safety, and compliance with existing frameworks is crucial for the sustainable adoption of new systems.
Regulatory Compliance
The FDA and EMA are actively evaluating AI applications in clinical research. Sponsors must ensure that AI-driven decisions, from patient recruitment to endpoint analysis, are traceable and reproducible. Models must be validated, and their performance must be monitored over time to meet regulatory expectations for clinical trial data integrity.
Data Privacy and Security
AI relies on large datasets, often including sensitive patient information. Compliance with regulations such as HIPAA in the U.S. and GDPR in Europe is mandatory. Companies are increasingly adopting federated learning and secure data anonymization techniques to enable AI analytics without compromising privacy.
Algorithmic Bias
AI models can unintentionally reinforce existing biases if training data are unrepresentative of diverse populations. In clinical trials, biased algorithms could skew patient selection, outcomes, or safety signals. Ongoing monitoring and the curation of an inclusive dataset are essential to mitigate this risk.
Informed Consent and Transparency
Patients must understand how AI influences trial procedures, eligibility, and data handling. Clear communication and transparent consent processes are essential for maintaining trust, particularly in trials that utilize decentralized or AI-driven recruitment methods.
Ethical Oversight
Institutional review boards (IRBs) and ethics committees are beginning to incorporate AI considerations into their review processes. Sponsors should provide detailed documentation of AI algorithms, risk mitigation strategies, and the rationale for AI use in study design and monitoring.
Navigating these regulatory and ethical considerations ensures that AI adoption enhances trial efficiency without compromising patient safety, fairness, or compliance. A proactive approach builds trust with regulators, investigators, and participants while maximizing the transformative potential of AI in clinical research.
Future Outlook and Market Trends for AI in Clinical Trials
The market for Artificial Intelligence in clinical trials is experiencing robust growth. Valued at USD 1.3 billion in 2024, it is expected to expand at a CAGR of 12.04% from 2025 to 2032, reaching USD 3.3 billion by 2032. This surge is largely driven by the rising global burden of chronic diseases such as diabetes, cardiovascular conditions, respiratory disorders, and cancer, which are increasing the demand for faster and more efficient drug development.
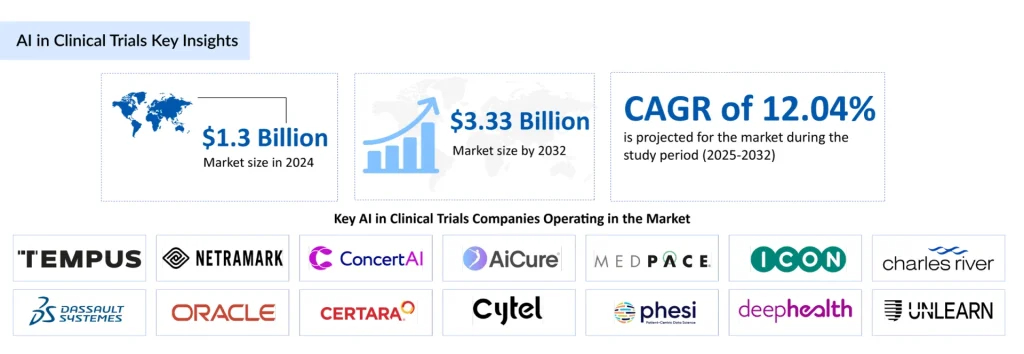
Strategic collaborations and partnerships among pharmaceutical, biotechnology, and medical device companies are accelerating the adoption of AI-powered trial solutions. Companies such as TEMPUS, NetraMark, ConcertAI, AiCure, Medpace, ICON, Dassault Systèmes, Oracle, Certara, Cytel, Phesi, DeepHealth, Unlearn.ai, H1, TrialX, Suvoda, Risklick, Lokavant, and Research Solutions are leading innovation in this space. In March 2025, Suvoda launched Sofia, an AI-powered assistant designed to streamline clinical trial management by providing real-time, actionable insights to study teams. In May 2025, Avant Technologies and its JV partner, Ainnova Tech, announced acquisition talks to strengthen their presence in the AI-driven healthcare market.
North America is expected to maintain dominance in the AI in clinical trials market. Factors contributing to this include high prevalence of chronic diseases, substantial research and development investments, and the growing number of clinical trials. Regional collaborations and the development of advanced AI solutions are further driving adoption, making North America the largest market for AI-based clinical trial solutions through 2032.
The combination of technological advancement, increased investment, and rising clinical trial complexity ensures that AI will continue to transform drug development. The market outlook remains highly promising, positioning AI as an indispensable tool in the next generation of clinical research.
Also, learn how AI is revolutionizing cardiac diagnostics with unmatched accuracy: Read here: AI Stethoscope
The Transformative Future of AI in Clinical Trials
Artificial Intelligence is becoming the backbone of modern trial design, execution, and analysis. By harnessing predictive analytics, automated data handling, and patient-centric technologies, AI is enabling sponsors to accelerate timelines, reduce operational burdens, and make trials more responsive to the real-world needs of patients.
The next wave of innovation is likely to focus on integrating multimodal data, combining genomics, imaging, and wearable data streams to provide unprecedented insights into treatment responses. AI-driven decision support systems may soon allow real-time trial adjustments, improving both safety monitoring and endpoint accuracy while minimizing patient exposure to ineffective therapies.
Moreover, AI is paving the way for more inclusive and decentralized clinical trials. Remote monitoring, digital consent, and algorithmically optimized patient engagement strategies are redefining accessibility, helping reach underrepresented populations and ensuring equity in trial participation.
As technology matures and regulatory frameworks evolve, the synergy of AI, advanced analytics, and collaborative ecosystems will enable clinical trials to be faster, wiser, and more responsive than ever before. The future of drug development is poised to be not only more efficient but also more personalized, ultimately bringing safe and effective therapies to patients with unprecedented speed.

Downloads
Article in PDF
Recent Articles
- Guardant Health’s Shield Test Earns FDA Breakthrough Device Status; Reflow Medical Gains FDA De N...
- Beta Bionics Introduces iLet Bionic Pancreas with Abbott’s FreeStyle Libre 3 Integration; Merit M...
- FDA Clears Fujirebio’s Lumipulse® G Plasma Test for Detecting Alzheimer’s-Linked Amyloid Patholog...
- HR Pharmaceuticals Announced Collaboration with Poiesis Medical; Enovis Acquired Lima Corporate; ...
- Eli Lilly’s COVID-19 Antibody trial; Approval for Oriahnn; Gilead’s Remdesivir Clinic...



