New Player in Ulcerative Colitis Treatment: Pfizer’s Etrasimod Entry Counters BMS’ Zeposia
Oct 20, 2023
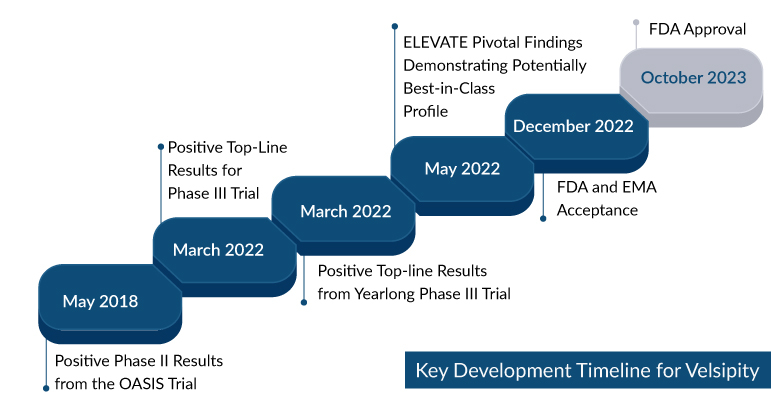
Bristol Myers Squibb’s Zeposia has lost its exclusive status as the sole S1P receptor modulator available for ulcerative colitis treatment. The FDA has granted approval for Pfizer’s ulcerative colitis medication, Etrasimod, under the brand name Velsipity. This decision comes after the publication of Phase III results in The Lancet, the company announced on October 13.
Pfizer’s etrasimod has received FDA approval as a daily oral treatment for adults with moderate to severe active ulcerative colitis. Velsipity becomes the second sphingosine-1-phosphate (S1P) medication to enter the ulcerative colitis treatment market, following the FDA’s approval of Bristol Myers Squibb’s Zeposia for the treatment of adults with moderately to severely active UC in May 2021. This approval signifies a profitable outcome for Pfizer, who acquired Arena Pharmaceuticals for $6.7 billion in March 2022. Etrasimod, the central asset in Pfizer’s acquisition of Arena in December 2021, was originally conceived as part of Pfizer’s lineup of immuno-inflammatory disease treatments.
Ulcerative colitis is an inflammatory bowel idiopathic condition, whose exact cause still remains unknown. As per DelveInsight, the total diagnosed prevalent cases of ulcerative colitis in the 7MM comprised approximately 3 million in 2022 and are projected to increase during the forecasted period. According to DelveInsight’s analysis, in the 7MM, the United States reported the highest prevalent cases of ulcerative colitis, which accounted for nearly 46% of the total 7MM cases, in the year 2022.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Emerging Therapies in the Chronic Pain Pipeline Transforming the Chronic Pain Market Landscape
- Antibody-Drug Conjugate and Big Pharmaceutical Companies
- Axovant rely on gene therapy; CAR-T treatments; Pfizer’s dacomitinib; Adaptimmune construct manif...
- Prostate Cancer Market Experiences an Influx of the Pharma Players Veering the Market Ahead
- Bristol-Myers Squibb’s Opdivo Approval; Mirati’s KRAS-inhibitor Adagrasib; J&J and Legend Bi...

Angela Hwang, Chief Commercial Officer and President of Global Biopharmaceuticals Business at Pfizer, announced, that VELSIPITY’s FDA approval is a major breakthrough for UC patients seeking steroid-free remission through a once-daily oral pill with a favorable benefit-risk profile. This marks a crucial milestone for those in need of advanced therapy for their chronic condition.
The FDA’s approval of VELSIPITY 2 mg once daily hinged on robust findings arising from the ELEVATE UC Phase III registration program. This program was divided into two crucial components, namely ELEVATE UC 52 and ELEVATE UC 12, both of which rigorously scrutinized the drug’s safety and efficacy in achieving clinical remission in patients suffering from ulcerative colitis. Notably, these patients had previously experienced inadequate responses or intolerances to conventional therapies, biologics, or Janus kinase (JAK) inhibitors. Of particular interest is the fact that nearly two-thirds of participants in ELEVATE UC 52 and ELEVATE UC 12 were new to biologic and JAK inhibitor treatments. Additionally, these studies stood out as the sole investigations within the advanced ulcerative colitis treatment landscape that included patients with isolated proctitis. Encouragingly, both ELEVATE UC 52 and ELEVATE UC 12 successfully achieved all primary and key secondary efficacy endpoints while maintaining a safety profile in line with earlier VELSIPITY research.
Dr. Michael Chiorean, Co-Director of the IBD Center at Swedish Medical Center and an investigator in the ELEVATE Registrational Program, emphasized that due to the unpredictable nature of UC, patients may go through multiple treatment options over time. In addition, some patients may feel hesitant about using injectable therapies, such as biologics. He underscored the importance of having innovative, effective alternatives like VELSIPITY for those patients who need advanced treatment and favor the convenience of a once-daily pill. VELSIPITY stands out as a well-established advanced treatment with a favorable benefit-risk profile.
According to Michael Osso, President and CEO of the Crohn’s & Colitis Foundation, ulcerative colitis affects each patient uniquely, and many people grappling with this condition contend with ongoing symptoms. The introduction of VELSIPITY as a new ulcerative colitis treatment widens the array of options for patients, and we are enthusiastic about witnessing its influence on individuals across the United States.
VELSIPITY exhibited excellent results in the ELEVATE UC 52 trial, with a 27.0% clinical remission rate at week 12 compared to 7.0% for the placebo group (a 20.0% difference, p<.001). The benefits persisted through week 52, as VELSIPITY achieved a 32.0% remission rate compared to the placebo’s 7.0% (a 26.0% difference, p<.001). In the ELEVATE UC 12 trial, VELSIPITY achieved clinical remission in 26.0% of patients at week 12, outperforming the 15.0% remission rate in the placebo group (an 11.0% difference, p<.05). Key secondary efficacy endpoints, such as endoscopic improvement and mucosal healing, were also successfully reached by week 12. The safety profile of VELSIPITY was consistent with previous studies, with common adverse reactions including headache, elevated liver tests, and dizziness, all with an incidence of 5% or higher.
Additionally, the study reported that adverse events occurred in 71% of patients receiving etrasimod in the 52 UC trial, while 56% of those on a placebo reported similar events. In the 12 UC trial, adverse events were observed in 47% of patients across both groups, with no recorded incidents of mortality or malignancies.
Pfizer has also highlighted a possible advantage in terms of tolerability for Velsipity, particularly regarding specific cardiovascular side effects. During the initial 12 weeks of the study, there were three instances of patients discontinuing treatment due to a slow heartbeat associated with Velsipity. However, Pfizer clarified that these adverse events were not categorized as serious.
Both the Velsipity and Zeposia labels contain the same cautionary note regarding bradyarrhythmia, which mandates patients to undergo an ECG test before initiating treatment. Nevertheless, in comparison to Zeposia, Velsipity doesn’t call for dosing titration. Pfizer and BMS have their sights set on broadening the application of their S1P drugs to address other autoimmune conditions, including Crohn’s disease, the alternative variant of inflammatory bowel disease.
Pfizer has cast a wider net than just Zeposia. Velsipity’s one-year remission rate of 32% is on par with several biologics available. Pfizer advocates for Velsipity to be recognized as a leading novel treatment, following the use of conventional 5-ASA therapies or steroids.
With time ulcerative colitis landscape is evolving and the treatment goals continue to shift hence the dynamics of the ulcerative colitis therapeutic market are anticipated to change in the coming years owing to the improvement in the rise in number of healthcare spending across the world. Major Pharma giants are thoroughly working toward the development of new ulcerative colitis treatment therapies, in order to provide better relief for the symptoms and hence improve the Quality of life (QoL) of patients with ulcerative colitis. The major goals for gastroenterological indications are clinical remission, clinical response, and mucosal healing. Key players such as AbbVie [Skyrizi (Risankizumab)], Eli Lilly and Company [Mirikizumab (LY-3074828)], Janssen (Johnson & Johnson) [Tremfya (Guselkumab)], Reistone Biopharma [SHR0302] Landos Biopharma (BT-11), and several others in the clinical development stage for ulcerative colitis treatment will lead to a significant increase in the market size during the forecast period [2023–2032]. Apart from this several other companies like Bristol-Myers Squibb, Pfizer, Connect Biopharma, and others are also investigating their products for ulcerative colitis treatment.

Downloads
Article in PDF
Recent Articles
- Nunaps raises USD 4.2M; Arcutis files for USD 100M IPO
- Takeda acquisition on Shire raises its share by 25 percent
- EC grants; Pfizer cuts; 27 medicines sold; Keytruda nabs
- Bristol Mayer Squibb — The Undisputed Leader in the Molecular Glue Arena
- GSK Is All Set To Launch Respiratory Syncytial Virus Vaccine… Now Who’s Next?