Alternative Therapeutic Strategies Beyond HSCT: Reimagining Curative Options in Hematologic Disease
Feb 16, 2026
Table of Contents
Summary
- HSCT is a potentially curative treatment for hematologic malignancies (acute leukemias, lymphomas, multiple myeloma) and bone marrow failure syndromes.
- In 2025, approximately 26,000 HSCT cases were reported in the 7MM, with Multiple Myeloma/Plasma Cell Dyscrasias having the largest patient pool in the US.
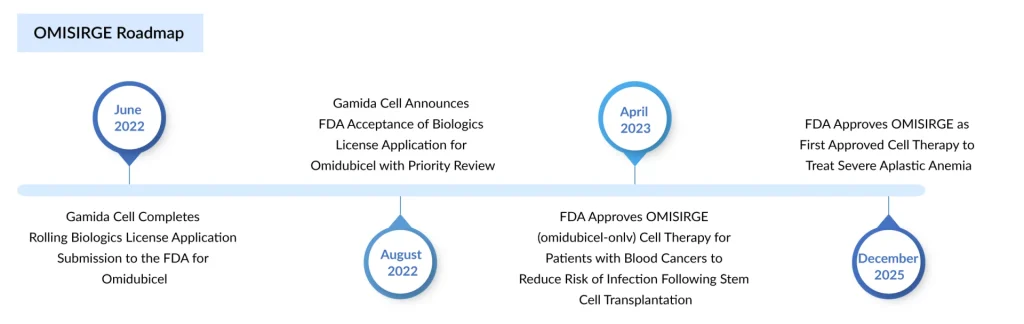
- FDA-approved Gamida Cell’s OMISIRGE in April 2023 for adults/adolescents (≥12 years) with hematologic malignancies undergoing cord blood transplantation. In December 2025, it became the first HSCT option specifically approved for severe aplastic anemia in patients aged 6+ who lack suitable matched donors and have undergone reduced-intensity conditioning.
- Cordex Biologics’ ZEMCELPRO received conditional marketing authorization from the European Commission in August 2025.
- Several key developers, including Orca Bio (Orca-T and Orca-Q) and Stratus Therapeutics (ST-101), are advancing treatments intended to replace HSCT.
Hematopoietic stem cell transplantation (HSCT) has been a cornerstone of treatment for a wide range of hematologic malignancies and select non‑malignant disorders for decades. It offers a potentially curative option for conditions such as acute leukemias, myelodysplastic syndromes, lymphomas, multiple myeloma, and various bone marrow failure syndromes. In 2025, the total number of HSCT cases in the 7MM was approximately 26,000, reports DelveInsight. Among the considered indications, Multiple Myeloma/Plasma Cell Dyscrasias (PCDs) had the largest pool in the US.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- BMS’s LPA1 Antagonist; Alnylam’s KARDIA-1 Phase 2 Study; Day One Biopharma Sought FDA Appro...
- FDA Approves Omeros’ YARTEMLEA: A Breakthrough for Stem Cell Transplant Patients Facing TA-TMA
- PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboratio...
- Actinium Announces Phase III SIERRA Trial Results; FDA Approves Apellis’s Geographic Atrophy Drug...
- Zenas BioPharma’s Obexelimab Delivers Positive Phase 3 Results in IgG4-RD; Sanofi Secures US Prio...
Global activity has expanded steadily, with more than 80,000 HSCT procedures reported annually worldwide and over 93,000 transplants performed in 2024 alone, encompassing both autologous and allogeneic procedures. In Europe, more than 47,000 hematopoietic cell transplants were carried out in 2023 across nearly 700 centers, underscoring the maturity and scale of this therapeutic modality.
Yet, despite its curative potential, HSCT is far from an ideal solution for many patients. It is associated with significant treatment‑related morbidity and mortality, complex logistics, and stringent eligibility criteria. Sadaf Javed, Manager of Forecasting and Analytics at DelveInsight, noted that a significant unmet need remains for patients undergoing conventional allo-HSCT, which may require a trade-off between graft-versus-leukemia (GvL) and toxicity. This trade-off often interferes with the success of the transplant. And oftentimes, despite success in many patients, the curative potential of allo-HSCT can be undermined by GvHD, infection, and relapse because there is no standardized approach to prevent them.
Why Look Beyond Conventional HSCT?
Standard allogeneic HSCT relies on donor‑derived hematopoietic stem cells (HSCs) and the graft‑versus‑leukemia (GvL) effect, but that benefit comes at the cost of substantial toxicity. Key limitations include:
- Graft‑versus‑host disease (GvHD): A major cause of morbidity and mortality, chronic GvHD can severely compromise quality of life and long‑term outcomes.
- Infections and delayed immune reconstitution: Myeloablative conditioning and slow engraftment, especially with cord blood grafts, expose patients to prolonged cytopenias and life‑threatening infections.
- Relapse risk: Even after a technically successful transplant, relapse remains a leading cause of treatment failure in many high‑risk malignancies.
- Donor availability and equity of access: Patients without HLA‑matched donors or from underrepresented ethnic groups often have limited access to standard allo‑HSCT.
- Eligibility constraints: Age, comorbidities, organ dysfunction, and performance status exclude a substantial proportion of patients from intensive transplant regimens.
These challenges are reflected in the epidemiology: HSCT numbers continue to rise globally, but access remains uneven, and outcomes vary significantly across regions and transplant types. Consequently, there is a strong clinical and commercial imperative for strategies that can maintain or improve curative potential while reducing toxicity and expanding eligibility.
Expanded Cord Blood–Derived Therapies: Closing Donor Gaps and Accelerating Engraftment
Expanded cord blood–derived therapies offer a promising solution to donor shortages by providing a more universally accessible stem cell source. Advances in ex vivo expansion techniques significantly increase cell counts, helping overcome traditional limitations of cord blood units. These therapies also promote faster engraftment, reducing complications and improving patient outcomes. Currently, there are two approved HSCT drugs: Gamida Cell’s OMISIRGE and Cordex Biologics’ ZEMCELPRO.
OMISIRGE: Making Cord Blood Transplant Faster and Safer
Omidubicel‑onlv (OMISIRGE, Gamida Cell) is an FDA‑approved, nicotinamide‑expanded, allogeneic cord blood–derived hematopoietic progenitor cell therapy. In April 2023, it was approved for adults and adolescents (≥12 years) with hematologic malignancies scheduled for umbilical cord blood transplantation after myeloablative conditioning, specifically to reduce the time to neutrophil recovery and the incidence of infections.
Furthermore, in December 2025, the FDA authorized Gamida Cell’s OMISIRGE as the first hematopoietic stem cell transplant option specifically approved for patients with severe aplastic anemia. The updated indication permits its use in adults and children aged six and older who need a transplant after reduced-intensity conditioning but lack a suitable matched donor.
Omisirge consists of cord blood stem cells processed with nicotinamide (a vitamin B3 derivative) to improve engraftment and support recovery of blood and immune function. The approval was supported by results from an ongoing open-label trial in which 12 of 14 patients demonstrated rapid and durable neutrophil engraftment, with a median recovery time of 11 days.
Functionally, OMISIRGE does not discard the concept of transplant; it still requires conditioning and a cord blood graft, but it redefines the risk profile of cord blood–based allogeneic therapy. For patients without suitable matched donors, especially from underrepresented populations, this expanded graft can make a potentially curative transplant more accessible and safer.
ZEMCELPRO: Expanding Access for “No‑Donor” Patients in Europe
ZEMCELPRO (UM171 Cell Therapy, Cordex Biologics, an ExCellThera company) advances a similar therapeutic concept in Europe. In August 2025, the European Commission granted conditional marketing authorization for its use in adults with hematologic malignancies who need an allogeneic HSCT after myeloablative conditioning but lack any other suitable donor cell source.
This therapy is a personalized, cryopreserved product generated from a single cord blood unit. It includes a UM171-expanded CD34+ fraction (dorocubicel), which enriches for true stem and progenitor cell populations, and an unexpanded CD34- fraction that preserves supportive immune cell subsets.
By significantly increasing the number of transplantable stem cells available from cord blood, ZEMCELPRO is designed to broaden access to donor-derived transplantation for patients who would otherwise lack viable donor options, while preserving the curative intent of allogeneic therapy. Functionally, it serves as an intermediary between traditional HSCT and emerging cell-therapy platforms: grounded in transplant biology, yet reshaping how donor scarcity and engraftment challenges are addressed.
According to Sadaf, ZEMCELPRO is positioned to substantially expand access to allogeneic stem cell transplantation, thereby addressing a critical bottleneck in the treatment of high-risk hematologic malignancies, such as leukemias and myelodysplastic syndromes. Its adoption could meaningfully expand the eligible patient pool and improve treatment pathways in settings where donor availability has historically constrained outcomes.
High‑Precision Allogeneic T‑Cell Immunotherapies: Re‑Engineering the Graft
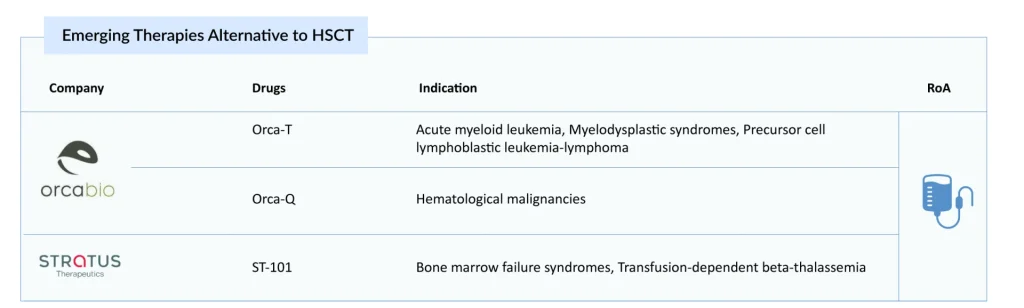
Perhaps the most transformative “beyond HSCT” approaches are high‑precision allogeneic T‑cell immunotherapies that deconstruct the traditional graft and reassemble it in a controlled fashion. Key players, including Orca Bio (Orca-T, Orca-Q) and Stratus Therapeutics (ST-101), are developing therapies as alternatives to HSCT.
Orca-T: Redefining Allogeneic Transplant Through Treg‑Based Precision
Orca-T is an investigational, donor-derived T-cell therapy being studied for use in several blood cancers and is currently in a Phase III clinical trial. Designed as a potential alternative to traditional allogeneic hematopoietic cell transplantation (allo-HCT), the product includes CD34+ stem cells, regulatory T cells, and conventional T cells collected from the peripheral blood of matched donors. In contrast to standard allo-HCT, Orca-T delivers these key cell populations in a staged manner. This sequencing allows the regulatory T cells to enter solid organs first and establish an immune-protective environment before the conventional T cells are infused.
In March 2025, Orca Bio reported positive top-line results from the pivotal Phase III Precision-T trial of Orca-T, which enrolled patients with AML, ALL, high-risk MDS, and mixed-phenotype acute leukemia (MPAL). Orca‑T is currently under FDA Priority Review with a target action date of April 6, 2026, positioning it as a potential first‑in‑class HSCT alternative in the near term.
According to Aparna Thakur, Assistant Project Manager, Forecasting and Analytics at DelveInsight, if approved, Orca‑T would represent a fundamental shift: the “transplant” becomes a precision‑engineered cell therapy where the immune effector composition, timing, and dose are deliberately designed, rather than accepted as an unavoidable by‑product of donor graft biology.
Orca-Q and the Move Toward Haploidentical Flexibility
Orca‑Q, a second‑generation candidate from Orca Bio, is being developed to extend this paradigm to haploidentical (partially matched) donors. Unlike Orca‑T, which is intended primarily for HLA‑matched donors, Orca‑Q is designed to deliver similar benefits without requiring a fully matched donor, potentially broadening access to high‑precision grafts for patients who currently rely on more toxic or high‑risk haploidentical transplant approaches. It is currently being evaluated in a Phase I/II clinical trial.
ST-101: Preclinical Progress Toward Universal HSCT Replacement
Several preclinical companies are developing on-demand hematopoietic stem cell therapies to replace conventional transplant procedures. Stratus Therapeutics’ lead candidate, ST-101, aims to serve as an alternative to traditional transplants. The product comprises both Prime HSCs™ and Prime HPCs™, supporting rapid immune recovery and durable, long-term engraftment. ST-101 provides a substantially higher dose of true HSCs than standard grafts and is produced with virtually no T-cells, thereby minimizing the risk of GvHD. The company’s initial clinical targets are patients with acquired or inherited bone marrow failure syndromes (BMFS) and individuals with transfusion-dependent beta thalassemia (TDT), with plans to broaden its use to all conditions currently treated with HSCT.
Conclusion
HSCT will remain a foundational therapy for hematologic malignancies and selected non‑malignant diseases for the foreseeable future. However, its role is poised to evolve. As expanded grafts such as OMISIRGE and ZEMCELPRO, high‑precision immunotherapies such as Orca‑T and Orca‑Q, and emerging on‑demand HSC platforms mature, the field is likely to shift from a single dominant curative modality to a portfolio of curative strategies tailored to patient biology, risk profile, and health‑system capabilities.In that future HSCT treatment landscape, “alternative therapeutic strategies beyond HSCT” are not merely competitors to transplant; they are integral components of a re‑imagined, more precise, and more equitable curative ecosystem in hematology, one that aims to preserve the life‑saving benefits of HSCT while overcoming its most persistent limitations.

Downloads
Article in PDF
Recent Articles
- PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboratio...
- BMS’s LPA1 Antagonist; Alnylam’s KARDIA-1 Phase 2 Study; Day One Biopharma Sought FDA Appro...
- WASKYRA Wins FDA Approval, Marking a Historic Leap for Rare Disease Gene Therapies
- Zenas BioPharma’s Obexelimab Delivers Positive Phase 3 Results in IgG4-RD; Sanofi Secures US Prio...
- FDA Approves Omeros’ YARTEMLEA: A Breakthrough for Stem Cell Transplant Patients Facing TA-TMA




