Can Remternetug Solve the Puzzle of Alzheimer’s?
Feb 12, 2025
Table of Contents
Alzheimer’s disease has become one of the most pressing global health challenges, with its prevalence rising dramatically over the past few years. As the aging population grows, so does the burden of this neurodegenerative disorder, placing immense strain on healthcare systems worldwide. The Alzheimer’s drug market has expanded significantly, driven by the urgent need for effective treatments to slow disease progression and improve patient outcomes.
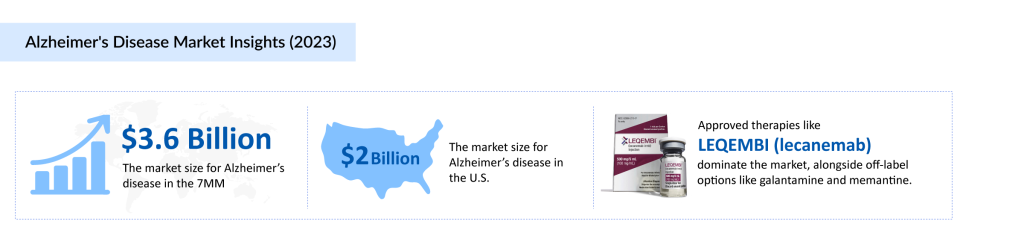
Currently, the Alzheimer’s disease treatment landscape is seeing a surge in approved therapies, with LEQEMBI making notable strides. From October to December 2024, LEQEMBI generated approximately $87.4 million, further establishing its presence in the market. Other than that, pharmaceutical companies are racing to develop groundbreaking therapies, with numerous clinical trials underway. From monoclonal antibodies targeting amyloid plaques to novel mechanisms addressing tau pathology, every contender in the space aims to crack the code of this devastating disease. Among the most promising candidates is Remternetug, a late-phase III investigational therapy that has garnered significant attention for its potential to redefine Alzheimer’s treatment. Could this drug be the breakthrough the world has been waiting for?
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Pfizer’s COVID-19 Vaccine; Calliditas’ NefIgArd trial; ViiV’s HIV Prevention Tr...
- KEY CLINICAL OUTCOMES
- LEQEMBI Intravenous Infusion Approval; Novartis’ Presented Updates on Lutathera; FDA Accept...
- Alzheimer’s drug fails! Is it time to move on?
- GHO to advance healthcare in Europe; Truvian nets USD 27.1 Mn; Lilly plans its diabetes portfolio...
Remternetug: A Journey Through Clinical Development
Remternetug, previously known as LY3372993, is an investigational monoclonal antibody developed by Eli Lilly and Company. Designed to target pyroglutamated amyloid-beta (Aβ), a key component of amyloid plaques in Alzheimer’s disease, Remternetug follows a similar mechanism to donanemab, Lilly’s FDA-approved antibody. A series of clinical trials have marked its development focused on assessing its safety, efficacy, and potential to modify the course of Alzheimer’s disease. As research advances, remternetug FDA approval stands as a promising contender in the race to combat this devastating neurodegenerative disease.
Remternetug Clinical Trials: The Initial Phase
In November 2018, Eli Lilly initiated a Phase I trial to compare remternetug to placebo in 100 healthy adults and individuals with mild cognitive impairment (MCI) due to Alzheimer’s disease or mild to moderate Alzheimer’s disease. Participants received intravenous infusions of single or multiple escalating doses, with assessments focusing on adverse events, blood exposure levels, and the drug’s impact on brain amyloid as measured by florbetapir PET scans. However, in April 2019, after enrolling 36 healthy adults, the trial was terminated without enrolling any Alzheimer’s disease patients, and the results were not publicly disclosed.
In July 2020, a new Phase I study commenced, enrolling 30 participants with clinically diagnosed MCI due to Alzheimer’s disease or Alzheimer’s disease dementia. They received multiple escalating doses of remternetug or placebo over nine months. The primary outcome was the number of serious adverse events, while secondary outcomes included pharmacokinetics and changes in brain amyloid levels from baseline to week 25. In 2021, the study expanded to include 32 healthy adults of first-generation Japanese origin receiving a single infusion. Enrollment was later increased to 224 participants, the treatment period was extended to 61 weeks, and subcutaneous dosing was added. This trial was conducted at multiple U.S. and Japan sites, with completion in November 2024.
Advancement to Phase III Trials
In August 2022, Eli Lilly initiated the Phase III TRAILRUNNER-ALZ1 trial to evaluate Remternetug as a potential breakthrough in the Alzheimer’s disease treatment landscape. The study initially aimed to randomize 400 individuals with early symptomatic Alzheimer’s disease to receive Remternetug or placebo for one year, administered either intravenously or subcutaneously. The primary outcome focuses on amyloid plaque clearance, a critical target in Alzheimer’s disease therapies, while secondary outcomes assess pharmacokinetics and the presence of anti-drug antibodies.
As the Alzheimer’s disease drug market continues to evolve, the trial expanded in 2023, increasing enrollment to 600 in the placebo-controlled arm and 974 in the safety cohort, adding additional sites in the U.S. and Japan. A blinded extension phase allows Remternetug recipients to switch to placebo and vice versa, ensuring a comprehensive assessment of long-term effects. The blinded portion of the study concluded in June 2024, with overall completion expected in March 2026, marking a significant step forward in advancing Alzheimer’s disease therapies.
Recent Developments
In October 2024, the Phase III TRAILRUNNER-ALZ3 study was initiated to evaluate Remternetug as a potential breakthrough in the Alzheimer’s disease treatment landscape. The trial aims to enroll 1,200 participants with early Alzheimer’s disease, administering subcutaneous injections of Remternetug or placebo over 18 months, followed by a blinded observation period. The primary endpoint focuses on time to progression on the Clinical Dementia Rating (CDR) scale, a key measure in assessing disease advancement. Secondary outcomes include cognitive, behavioral, and functional assessments, along with serum antibody concentrations and adverse event monitoring, critical for evaluating Alzheimer’s disease therapies.
As the Alzheimer’s disease drug market advances, this trial requires plasma pTau217 levels consistent with amyloid pathology, ensuring targeted enrollment of individuals with minimal cognitive or functional impairment. The study includes amyloid and tau PET substudies and an open-label extension, with completion expected in 2029. Remternetug has already demonstrated significant amyloid plaque reduction in early-stage trials, with no treatment-emergent anti-drug antibodies detected. As it progresses through Phase III trials, Remternetug stands as a promising disease-modifying therapy, potentially transforming the Alzheimer’s disease treatment landscape.
Efficacy and Safety Profile
The TRAILRUNNER-ALZ1 study, launched in August 2022, is a clinical trial designed to evaluate the efficacy and safety of Remternetug (LY3372993) in individuals with early Alzheimer’s disease. The study aims to enroll 1,667 participants aged 60–85 years, with the trial expected to run until March 2026. Participants may receive either Remternetug or a placebo, with a treatment duration of up to two years. The trial primarily assesses amyloid plaque clearance, along with safety measures, including monitoring for adverse events and the presence of anti-drug antibodies.
Building on this, the TRAILRUNNER-ALZ3 study, initiated in October 2024, focuses exclusively on the efficacy of Remternetug in early Alzheimer’s disease treatment. By targeting amyloid pathology and evaluating its potential as a disease-modifying therapy, Remternetug is positioning itself as a promising candidate in the Alzheimer’s disease drug market. With its ability to significantly reduce amyloid plaques, ongoing trials will determine if Remternetug can offer a safer and more effective alternative in the evolving Alzheimer’s disease treatment scenario.
Alzheimer’s Disease Current Market Scenario
The Alzheimer’s disease drug landscape has long been dominated by symptom-modifying treatments, primarily targeting neurotransmitter regulation through acetylcholinesterase inhibitors (AChEIs) like donepezil, rivastigmine, and galantamine, as well as memantine, an NMDA receptor antagonist. While these medications provide temporary cognitive benefits, they fail to halt disease progression, necessitating frequent reassessment of treatment efficacy. Additionally, side effects and varying benefit-risk ratios have led to inconsistent reimbursement policies across different regions, limiting accessibility for some patients.
However, this landscape is undergoing a transformational shift. The recent FDA approvals of LEQEMBI, KISUNLA, and REXULTI mark a growing focus on disease-modifying therapies (DMTs) aimed at reducing amyloid plaques rather than simply managing symptoms. These advancements signal a move toward more personalized and targeted treatment strategies, fueling both market expansion and investment in next-generation therapies.
How Remternetug Stands Out in the Current Alzheimer’s Therapeutic Landscape
Amid this evolving treatment paradigm, Remternetug emerges as a strong contender in the Alzheimer’s disease drug market. As the successor to Donanemab, Remternetug represents a major breakthrough in the evolving Alzheimer’s disease treatment scenario. Among emerging Alzheimer’s disease drugs, it stands out due to its ability to rapidly and effectively clear amyloid plaques, a key pathological feature of the disease. Phase I clinical trials demonstrated a strong dose-dependent response, with higher doses (700–2800 mg) leading to significant reductions in amyloid burden. By day 169, 18 out of 24 treated patients achieved amyloid clearance, underscoring its potential as a game-changing therapy in the Alzheimer’s disease treatment landscape.
With its Phase III trial expected to conclude by October 2025, Remternetug is positioned to redefine Alzheimer’s disease therapies. Its ability to deliver rapid and sustained amyloid clearance could make it one of the most effective Alzheimer’s disease drugs available. If successful, it may offer a more efficient and targeted approach compared to existing monoclonal antibodies, further strengthening its role in the next generation of treatment options for Alzheimer’s disease.
The Future of Alzheimer’s Treatment: A Step Closer to a Breakthrough
With decades of research and countless setbacks, the fight against Alzheimer’s has been defined by perseverance and incremental progress. However, the emergence of disease-modifying therapies signals a new era—one that goes beyond symptom management to target the underlying pathology. Remternetug, with its robust amyloid-clearing potential and promising clinical data, stands at the forefront of this revolution. If its Phase III trials confirm its efficacy and safety, it could redefine the treatment paradigm, offering patients and caregivers a tangible step toward slowing disease progression. As the Alzheimer’s disease landscape evolves, Remternetug has the potential to not only be a breakthrough therapy but a beacon of hope in the ongoing battle against neurodegeneration.

Downloads
Article in PDF
Recent Articles
- Valo Health receives $110M Series B Cognito’s therapy slows Alzheimer’s disease Venty...
- The Automated Eye: How AI-Driven Diagnostics are Reshaping the Future of Medical Imaging
- Does coconut oil help prevent dementia?
- Ipsen to Acquire Albireo; Chiesi Farmaceutici to Buy Amryt Pharma; Takeda Presents Phase III Resu...
- Biogen’s Aduhelm; FDA Approves Sanofi’s Enjaymo; NHS & Orchard Signs a Deal; Bri...