Iovance’s Breakthrough: Amtagvi Paves the Way for Cell Therapies in Solid Tumors
Feb 26, 2024
The category of T-cell therapy, which revolutionized the management of specific blood cancers, has now extended its impact to solid tumor treatment with the approval from the FDA of a groundbreaking immunotherapy pioneered by Iovance Biotherapeutics. Known as Amtagvi or lifileucel, this medication marks the debut of personalized tumor-infiltrating lymphocyte (TIL) therapy in the market.
WuXi Advanced Therapies, a company fully owned by WuXi AppTec, has revealed that its Philadelphia facility has gained approval from the FDA to commence analytical testing and manufacturing of Amtagvi for Iovance. This follows Iovance’s receipt of FDA accelerated approval for its Biologics License Application (BLA) on February 16, 2024.
Amtagvi, an autologous T-cell therapy derived from tumors, is designed for use in adult patients with advanced melanoma who cannot be removed surgically or have spread, who have already undergone treatment with a PD-1 blocking antibody. Additionally, if they test positive for the BRAF V600 mutation, treatment with a BRAF inhibitor, with or without a MEK inhibitor, is recommended. This approval for Amtagvi is granted under an accelerated process, considering its effectiveness in overall response rate (ORR) and duration of response. Iovance is also progressing with the Phase III trial TILVANCE-301 to further validate its clinical benefits.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Exosomes: Tiny Messengers with Big Potential in Medical Science
- 5 Most Promising CAR T-cell Therapies for Multiple Myeloma in Development
- FDA Approves Genentech’s GAZYVA for Lupus Nephritis; Glaukos’ EPIOXA Wins FDA Approval for Kerato...
- Evolving Landscape of Anti-GPC3 Targeted Therapies: ADCs, CAR-T Cells, and Beyond
- Opportunities and Challenges for Cell and Gene Therapies
Amtagvi stands as the first and only one-time, personalized T-cell treatment endorsed by the FDA for combating solid tumor cancer. Its conceptual framework introduces a novel avenue for cell therapy, leveraging patient-tailored TIL cells. These cells, generated by the immune system upon cancer detection, are tasked with identifying, targeting, and eliminating cancerous cells. Specifically attuned to unique tumor markers present in an individual’s cancer cells, TIL cells become compromised in their anti-cancer function as the disease progresses.
Amtagvi is manufactured through an exclusive method designed to gather and multiply a patient’s T cells from a segment of their tumor. Following this process, billions of these T-cells are reintroduced into the patient’s body to combat their cancer. Authorized Treatment Centers (ATCs) will oversee the administration of Amtagvi to patients, alongside lymphodepletion and a brief period of high-dose PROLEUKIN® (aldesleukin) as part of their treatment plan.
“We view the accelerated approval of Amtagvi as the initial stride towards fulfilling Iovance’s vision of introducing the next era of cell therapy, making this groundbreaking treatment accessible to those battling advanced solid tumors,” stated Frederick Vogt, Ph.D., J.D., serving as Interim Chief Executive Officer and President of Iovance. “With a profound scarcity of effective treatments within the advanced melanoma community, we take pride in offering a tailored, one-time therapeutic alternative for these individuals. Our dedication remains unwavering as we persist in our efforts to tackle further unmet medical necessities among patients with solid tumor malignancies, extending the reach of our innovative cell therapies to encompass more individuals grappling with melanoma and other forms of cancer.”
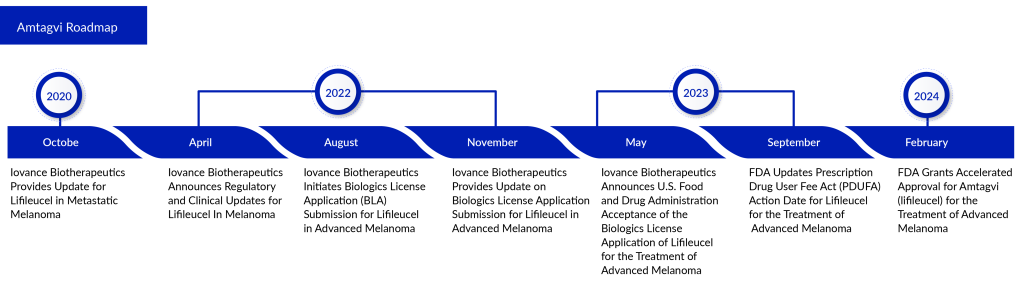
With this approval, the Philadelphia location of WuXi ATU becomes the first external manufacturing site in the United States, and the first third-party contract testing, development, and manufacturing organization (CTDMO) sanctioned by the FDA for aiding in the commercial production and release of a personalized T cell therapy targeting solid tumor cancer.
Every year, around 8K individuals in the United States lose their lives to melanoma. Up until this point, there have been no treatments approved by the FDA for individuals with advanced melanoma whose condition worsened after their initial treatment with an immune checkpoint inhibitor and, if suitable, targeted therapy.
Samantha R. Guild, J.D., President of AIM at Melanoma Foundation, expressed optimism about the approval of Amtagvi, seeing it as a beacon of hope for individuals battling advanced melanoma after exhausting initial standard treatments. She highlighted the limited effectiveness of current options for many patients. Guild emphasized that this singular cell therapy signifies a significant advancement in melanoma treatment, offering great promise to transform the landscape of care for those urgently requiring more therapeutic choices.
The FDA’s authorization is rooted in the safety and effectiveness findings from the C-144-01 clinical trial. This trial, conducted across multiple centers worldwide, looked into Amtagvi’s effects on patients with advanced melanoma who had previously undergone anti-PD-1 therapy and targeted treatment where applicable. Amtagvi displayed significant and lasting improvements. The main analysis, involving 73 patients from Cohort 4 who received the recommended dosage of Amtagvi from an approved production site, revealed that 31.5% achieved a positive response according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1), with no defined median duration of response within an 18.6-month follow-up period (43.5% of these responses lasted over 12 months). Moreover, the combined efficacy analysis considered a total of 153 patients from both Cohort 4 and Cohort 2. Within this group, 31.4% exhibited an objective response per RECIST 1.1, with no specified median duration of response during a 21.5-month follow-up period (54.2% of these responses lasted over 12 months). Detailed findings from the C-144-01 clinical trial are available in The Journal for ImmunoTherapy of Cancer.
Iovance has established a wholesale acquisition cost of $515,000 per patient for the one-time treatment. This price slightly exceeds the current costs of existing CAR-T cell therapies, which typically range around $500,000, or lower, for individuals with blood cancer. The pricing decision was influenced by Amtagvi’s value as the inaugural drug sanctioned for this post-PD-1 melanoma context, in addition to Iovance’s examination of pertinent benchmarks.
“This significant FDA approval marks substantial progress in TIL cell therapy since our initial demonstration that TIL cells extracted from individuals with metastatic melanoma could be cultured and reintroduced to the patient to facilitate cancer regression,” stated Dr. Steven Rosenberg, Chief of the Surgery Branch at the National Cancer Institute and a trailblazer in TIL and immunotherapy. “It represents a pivotal moment for the research community as a whole and encourages further exploration of TIL cell therapy in various other solid tumor types.”
Before Amtagvi, CAR-T therapies were primarily effective against certain blood cancers, partially due to the absence of suitable cell-surface biomarkers on solid tumors for CAR-T cells to target. A TIL therapy addresses this challenge by leveraging TIL cells, which are inherently designed to recognize cancer biomarkers.
Despite its innovative design, Amtagvi does have its shortcomings. To begin with, since Amtagvi utilizes TIL cells extracted from a patient’s own tumor, individuals who are unable to undergo surgery or those who do not have adequate resected tumor tissue may not qualify for this treatment. Additionally, the medication comes with a boxed warning due to risks of treatment-related fatalities, prolonged severe cytopenia, severe infection, as well as potential impacts on cardiopulmonary and kidney functions. Consequently, the drug is solely administered at specified treatment centers within hospital premises, requiring an inpatient setting. Furthermore, the FDA-approved label mandates rigorous monitoring of patients for adverse effects within an intensive care unit, with the presence of specialized medical professionals.
However, Vogt contended that Amtagvi’s boxed warning represents an improvement compared to the circumstances surrounding current CAR-T therapies. For instance, Amtagvi is not subject to a safety program mandated by the FDA, which is known as risk evaluation and mitigation strategies. Furthermore, the medication does not carry cautions regarding cytokine release syndrome or hemophagocytic lymphohistiocytosis, both of which are serious complications that can arise from CAR-T cell therapies.
Besides Amtagvi, Iovance is also developing another TIL therapy called LN-145, which is undergoing phase II trials for post-PD-1 non-small cell lung cancer. The pivotal IOV-LUN-202 trial might lead to accelerated approval.

Downloads
Article in PDF
Recent Articles
- FDA Approves Genentech’s GAZYVA for Lupus Nephritis; Glaukos’ EPIOXA Wins FDA Approval for Kerato...
- 10 Game-Changing Acute Myeloid Leukemia Drugs Revolutionizing Treatment
- FDA Grants Priority Review for Zolbetuximab BLA; FDA Traditional Approval for LEQEMBI for Alzheim...
- Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
- FDA Approves Xolair for Food Allergies; FDA Accelerated Approval for Iovance’s AMTAGVI; Astellas ...