Unveiling the Potential of TROP-2 Inhibitors: A New Frontier in Cancer Treatment
Mar 15, 2024
Table of Contents
Trophoblast cell surface antigen-2 (TROP-2) is a 35-kDa protein found on cell membranes, made of sugars and a protein chain, serving as a transmitter of calcium signals. It is produced by the TACSTD2 gene and shares a structural similarity with the epithelial cell adhesion molecule (EpCAM).
TROP2, although identified for its promising potential in cancer treatment some years ago, is now emerging as a significant protein target. With the rise of targeted therapies and immunotherapies, TROP2 has gained attention due to its crucial role in cancer cell proliferation and spread. Its increased presence in various cancers further solidifies its status as a valuable protein target.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- The Next Chapter in NSCLC Treatment Space: Recent Discoveries and Innovations
- Cilta-cel Demonstrates Prolonged and Deep Responses across Advanced Treatment Lines, Signifying P...
- Insights into the Evolving Landscape of Antibody-Drug Conjugate (ADC) & the Key Companies in...
- Next Generation Antibody-Drug Conjugate Therapeutics and Market Analysis
- Cervical Cancer Treatment: Navigating the Changing Landscape
The gene is active in typical human body tissues like the breast, cervix, kidney, lung, and pancreas, playing a role in embryonic growth. Additionally, it has been observed to increase its activity in various cancer types such as head and neck squamous cell carcinoma, gastric cancer, gallbladder cancer, cervical cancer, lung cancer, breast cancer, colon cancer, pancreatic cancer, ovarian carcinoma, and endometrioid endometrial carcinoma. This suggests that medications targeting TROP-2 might have the potential to address numerous types of cancer that currently lack effective treatments.
As per the European Society for Medical Oncology (ESMO), non-small cell lung cancer (NSCLC) stands as the predominant form of lung cancer, representing approximately 80─90% of all diagnosed cases of lung cancer. As per DelveInsight analysis, the United States had 228K incident cases of lung cancer in 2020, of which 194K were NSCLC cancer patients.
TROP-2 overexpression appears to correlate with a heightened likelihood of metastasis among patients afflicted with a range of cancer types (including oral squamous, thyroid, certain oesophageal, gastric, colorectal, pancreatic, ovarian, uterine, cervical, prostate, and urinary bladder cancers). Conversely, its upregulation is not observed in some other types (like head and neck cancers, and specific lung cancers such as lung adenosquamous and squamous cell carcinoma).
TROP-2 Inhibitors: Redefining the Landscape of Cancer Treatment
TROP-2 inhibitors have been under examination as prospective remedies for diverse cancer forms. The basis for inhibiting TROP-2 in cancer treatment stems from its heightened presence across various cancer types, correlating with aggressive tumor behavior, metastasis, and unfavorable prognoses. The objective is to interfere with cancer growth and advancement by pinpointing TROP-2 and disrupting associated signaling pathways. Strategies like monoclonal antibodies, ADCs, small molecule inhibitors, or CAR-T cell therapy are integrated into treatment protocols to selectively target cancer cells exhibiting elevated TROP-2 expression.
In clinical terms, the therapeutic promise of TROP-2 is highlighted by its higher expression in the majority of cancers compared to its sporadic expression in healthy tissues. This makes it a highly encouraging candidate for delivering cancer-killing substances specifically to cancer cells. This approach is already in use for various tumors, particularly in cases where the usual treatment choices are restricted (like TNBC and urothelial carcinoma) or ineffective (such as SCLC).
Monotherapy with TROP-2 Inhibitors: TROP-2 inhibitors have the potential to serve as independent treatments for specific cancers, especially those exhibiting elevated TROP-2 expression. These include monoclonal antibodies directed at TROP-2, ADCs designed to deliver toxic substances to cells expressing TROP-2, small molecule inhibitors that disrupt TROP-2 signaling, or CAR-T cell therapy aimed at TROP-2. These options can be used alone to hinder the growth of cancer cells and promote their demise.
Combination Therapy: TROP-2 inhibitors could potentially be paired with additional anticancer medications to boost their effectiveness. One option is to pair TROP-2-focused ADCs with either chemotherapy or immunotherapy drugs to create a stronger combined impact. Researchers may also investigate combinations with targeted treatments or radiotherapy to enhance the results of treatment.
CAR-T Cell Therapy: CAR-T cell therapy is the process of modifying a patient’s T cells genetically so that they produce chimeric antigen receptors (CARs) aimed at TROP-2. These modified T cells are designed to identify and eliminate cancer cells that have TROP-2. This therapy offers hope as a specific immunotherapy strategy for cancers where TROP-2 is mutated or excessively expressed.
TRODELVY: First FDA-approved Anti-TROP-2 ADC
TRODELVY (Sacituzumab govitecan), originally developed by Immunomedics and later acquired by Gilead Sciences in September 2020, is an antibody-drug conjugate. It consists of a humanized monoclonal antibody, hRS7 IgG1k, targeting TROP-2, combined with SN-38, an active form of irinotecan (IRI), which is a type of topoisomerase I inhibitor. The FDA approved TRODELVY in April 2020 for the treatment of metastatic triple-negative breast cancer (mTNBC) in adults who have undergone at least two prior therapies, marking it as the first FDA-approved ADC targeting TROP-2.
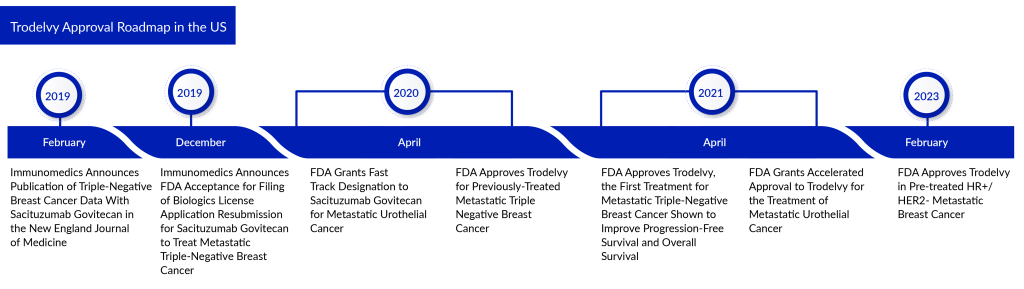
Further approvals followed: in April 2021, it gained FDA approval for use as a second-line treatment for triple-negative breast cancer and received accelerated approval for the second-line treatment of advanced urothelial carcinoma. Additionally, in February 2023, TRODELVY received FDA approval for the treatment of adults with hormone receptor-positive (HR+)/HER2-negative breast cancer who have already undergone endocrine-based therapy and at least two other systemic treatments in the metastatic setting.
Promising TROP-2 Inhibitors in Development
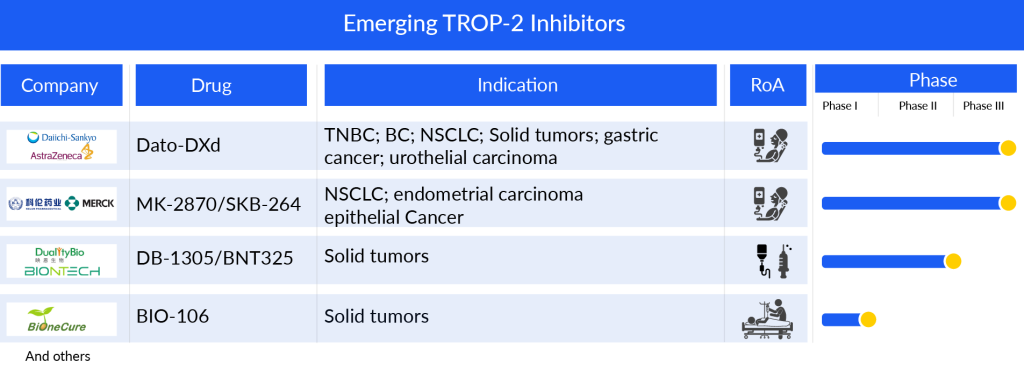
The pipeline of TROP-2 targeting inhibitors is robust and consists of various candidates in the clinical and preclinical phases including datopotamab deruxtecan (Dato-DXd) (Daiichi Sankyo/AstraZeneca), MK-2870/SKB-264 (Kelun Biotech/Merck), DB-1305/BNT325 (DualityBio/BioNTech), BIO-106 (BiOneCure Therapeutics), and others which are designed to target a wide range of cancers including non-small cell lung cancer, gastric cancer, urothelial carcinoma, epithelial cancer, bladder cancer, and others. Further approvals for TRODELVY and newer approvals for other candidates are expected to lead the TROP-2 inhibitors market.
Datopotamab deruxtecan (also known as Dato-DXd, DS-1062) is a TROP2-directed investigational ADC created using Daiichi Sankyo’s specialized DXd ADC technology. Dato-DXd comprises a humanized anti-TROP2 IgG1 monoclonal antibody linked to several topoisomerase I inhibitor payloads, specifically an exatecan derivative (DX-8951 derivative, DXd), connected through a tetrapeptide-based cleavable linker.
Recently, in February 2024, AstraZeneca and Daiichi Sankyo’s Biologics License Application (BLA) for Dato-DXd was accepted in the United States for treating adult patients with locally advanced or metastatic NSCLC who have previously undergone systemic therapy. The PDUFA date is set for the fourth quarter of 2024. The submission is grounded on findings from the pivotal TROPION-Lung01 Phase III trial. Moreover, Dato-DXd is being investigated in the NSCLC field in combination with immune checkpoint inhibitors. Beyond NSCLC, the drug is undergoing evaluation in several Phase III trials for breast cancer and Phase II and Phase I/II trials for gastric cancer, urothelial carcinoma, and various other solid tumors.
SKB264, an advanced ADC aimed at TROP2, has been developed by Merck in partnership with Sichuan Kelun-Biotech. It comprises a humanized monoclonal antibody directed at TROP-2, combined with a new topoisomerase I inhibitor connected through an enzymatically cleavable linker. This medication is currently undergoing Phase III trials for specific non-small cell lung cancer patients and certain individuals with previously treated endometrial carcinoma. Furthermore, Merck is also engaged in a Phase I/II study for MK-2870 targeting other advanced unresectable or metastatic solid tumors. Results from a Phase II extension investigation involving patients with locally advanced or metastatic NSCLC were shared at the 2023 ASCO annual conference.
DB-1305 is a specific ADC combining an anti-TROP-2 antibody with a new topoisomerase I inhibitor, P1021, connected by an enzymatically divided tetrapeptide linker. It is presently under investigation in a Phase I/II study involving individuals with advanced solid tumors.
As of January 2024, the FDA granted Fast Track Designation (FTD) to DB-1305 for treating patients with platinum-resistant ovarian epithelial cancer, fallopian tube cancer, or primary peritoneal cancer, who have undergone one to three earlier systemic treatment plans.
The anticipated launch of these emerging therapies are poised to transform the TROP-2 inhibitors market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the cancer market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
The Future Outlook of TROP-2 Inhibitors
In the targeted cancer therapies domain, TROP-2 inhibitors stand as heralds of a transformative future. With ongoing advancements in research and development, the outlook for TROP-2 inhibitors appears increasingly auspicious. These inhibitors hold the potential to revolutionize cancer treatment paradigms, offering tailored approaches that minimize adverse effects while maximizing therapeutic efficacy. As they progress through clinical trials, the horizon is rife with anticipation, envisioning a landscape where TROP-2 inhibitors become stalwart allies in the fight against cancer.
While Gilead currently holds the top spot in the TROP-2 targeted therapy market, it is not the sole contender. Close behind are Daiichi Sankyo and AstraZeneca, who have teamed up to create and bring to market the new TROP-2 antibody-drug conjugate, DS-1062. This development has the potential to enhance treatment protocols for lung, breast, and various other cancers. With AstraZeneca and Daiichi Sankyo intensifying the competition, Gilead has initiated trials for its own TROP-2 antibody-drug conjugate as an initial treatment for non-small cell lung cancer. These trials, supported by Merck, are exploring combination therapy with KEYTRUDA. Alongside this fierce rivalry among major pharmaceutical companies, smaller players in the industry have quietly been advancing their unique TROP-2 targeted therapies.
As the trajectory of TROP-2 inhibitors unfolds, their multifaceted applications paint a vibrant picture of the future of oncology. Not only do these inhibitors hold promise for traditional solid tumors, but their efficacy extends to various malignancies, including breast, lung, colorectal, and pancreatic cancers. Furthermore, the versatility of TROP-2 inhibitors opens doors to combination therapies, enhancing treatment outcomes through synergistic effects. This dynamic potential ignites optimism among researchers, clinicians, and patients alike, as they envision a future where TROP-2 inhibitors play a pivotal role in personalized, precise, and potent cancer treatments. As the journey continues toward regulatory approval and clinical integration, the future of TROP-2 inhibitors shines bright, poised to redefine the landscape of cancer care.

Downloads
Article in PDF
Recent Articles
- Antibody–Drug Conjugates: An Emerging Concept in Cancer Therapy
- ALLO-647 Integration in Lymphodepletion: Paving the Way for Enduring Responses and Safe CAR T cel...
- Antibody-Drug Conjugate: The Smart Biological Bomb
- PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboratio...
- Top 10 Expected Oncology Drug Launches in 2023



