Investigating the Pipeline for Ankylosing Spondylitis Treatment Landscape
Apr 11, 2022
Table of Contents
Ankylosing Spondylitis is a form of arthritis that primarily affects the spine. It causes severe inflammation of the spinal joints (vertebrae) that can lead to chronic pain and discomfort. The hallmark feature of Ankylosing Spondylitis is the involvement of sacroiliac (SI) joints during the Ankylosing Spondylitis progression. Currently, there is no permanent Ankylosing Spondylitis cure. But there are many Ankylosing Spondylitis treatment therapies available for symptomatic relief. Genetics plays an important role in ankylosing spondylitis occurrence, over 90% of patients with this condition have a particular genetic marker in their white blood cells, called HLA-B27. The HLA-B27 Ankylosing Spondylitis genetic marker appears to play a role in over 90% of the cases.
The primary Ankylosing Spondylitis risk factors responsible for the development of the disease are age, gender, other genes, and heredity. The disease tends to first appear in people between the ages of 17 and 45, though it has been known to affect people of all ages and genders. People with frequent infections of the gastrointestinal (GI) tract may be more predisposed to develop Ankylosing Spondylitis. HLA-B27 plays a major role in the Ankylosing Spondylitis pathogenesis, and it has recently been shown to profoundly affect the gut microbiome and its metabolites and the handling of bacteria during infection.
Downloads
Click Here To Get the Article in PDF
Ankylosing Spondylitis diagnosis criteria can be a little difficult because the condition develops slowly and there is no definite test. Currently, the modified New York classification has been used for Ankylosing Spondylitis for both research and clinical purposes. Factors responsible for Ankylosing Spondylitis diagnosis are physical exam, individual medical history, and a family history of Ankylosing Spondylitis, as well as blood work (including a test for HLA-B27), in addition to that, Ankylosing Spondylitis x ray also provide as a great tool for diagnosis. Ankylosing Spondylitis treatment includes NSAIDs, Sulfasalazine, Methotrexate, TNF Inhibitors, IL-17 Inhibitors, and many more therapies. Ankylosing Spondylitis prognosis may vary depending on the onset of disease symptoms, and it varies from person to person. As the disease progresses, pain and stiffness generally become more severe and regular. Ankylosing Spondylitis life expectancy can be considered somewhat similar to that of the general population if non-serious form of disease is present, on the contrary patients who have the most severe forms of the disease can experience serious complications.
Ankylosing Spondylitis Epidemiology and Statistics
In the United States, Ankylosing Spondylitis is almost undiagnosed in roughly 50% of cases because of its mild course. The delay between Ankylosing Spondylitis symptoms, onset and diagnosis has been estimated at approximately 13 years in the US. As per DelveInsight, Ankylosing Spondylitis prevalence in the 7MM countries was estimated to be around 2.2 million cases in the year 2021. The estimates suggest that diagnosed prevalent Ankylosing Spondylitis cases in the United States were 558K cases in 2021.
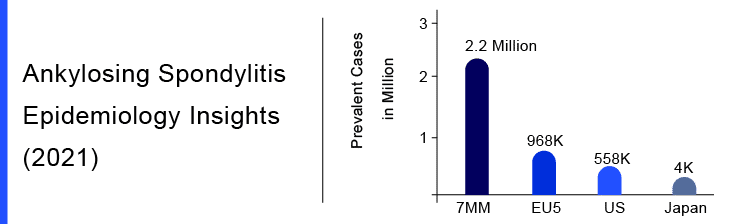
DelveInsight analysts indicate that there were nearly 968K Ankylosing Spondylitis cases in the EU-5 countries in 2021. According to the report, Germany accounted for the highest number of cases with 128K Ankylosing Spondylitis cases in 2021, followed by Spain and the United Kingdom. Also, the total diagnosed prevalent Ankylosing Spondylitis cases in Japan was found to be around 4K cases. DelveInsight estimates suggest that a higher diagnosed prevalence of the HLA-B27 gene type can be seen throughout the 7MM.
Ankylosing Spondylitis Market Insights
DelveInsight anticipates a huge surge in Ankylosing Spondylitis market size in the coming years owing to the increase in the prevalent Ankylosing Spondylitis patient population and the expected entry of novel emerging therapies with new mechanisms of action is contemplated to fuel the Ankylosing Spondylitis market. According to DelveInsight analysis, Ankylosing Spondylitis market size in the 7MM countries was estimated to be USD 4.2 Billion in 2021.

Many key pharmaceuticals that are proactively involved in producing Ankylosing Spondylitis drugs in the coming years include names such as Pfizer, UCB Biopharma, Fresenius Kabi, Shanghai Henlius Biotech, Fujifilm Kyowa Kirin Biologics, Hetero Biopharma, Bio-Thera Solutions, Amgen, CinnaGen, Zydus cadilla, Cadila Pharmaceuticals, Sandoz, Innovent Biologics, Torrent Pharmaceuticals, Cipla, Emcure Pharmaceuticals, Biogen, Boehringer Ingelheim, Zhejiang Hisun Pharmaceuticals, Biocad, AbbVie, Mycenax Biotech, Celltrion, Gilead Sciences, Amgen, Sun Pharma Global, Jiangsu HengRui Medicine Co., Ltd., Izana Bioscience, Suzhou Zelgen Biopharmaceuticals, Iltoo Pharma, Kinevant Sciences, Qyuns Therapeutics, Akeso Biopharma, Nimbus Therapeutics, Enzene Biosciences, Xbrane Biopharma, Dice molecules, whose key Ankylosing Spondylitis treatment therapies are expected to be launched in the coming years and can also serve as a major booster for Ankylosing Spondylitis treatment market.
On the contrary, the entry of biosimilar in the Ankylosing Spondylitis market will increase the competition and might hinder the market of both the approved and upcoming therapies if they are proven to be more effective and potential. It has been also observed that improved regulatory processes are also allowing the approval of biosimilars and making the process easier. Approval of biosimilar will result in a decline in sales demonstrating the increasing likelihood of high revenue loss following patent expiry of the already approved drugs.
Current Ankylosing Spondylitis Treatment Landscape
Ankylosing Spondylitis is an inflammatory disease that, over time, can cause some of the small bones in the spine (vertebrae) to fuse. This fusing makes the spine less flexible and can result in a hunched-forward posture. If ribs are affected, it can be difficult to breathe deeply. There is no permanent cure for the disease but Ankylosing Spondylitis treatment can lessen the symptoms and possibly slow the progression of the disease. Recent studies show that the newer biological Ankylosing Spondylitis medications can potentially slow disease progression in some people. Different people respond to different Ankylosing Spondylitis medications with varying levels of effectiveness. Thus, it may take time to find the most effective course of treatment for Ankylosing Spondylitis.
Ankylosing Spondylitis medication includes Non-steroidal Anti-Inflammatory Drugs (NSAIDs), Disease-modifying Antirheumatic Drugs (DMARDs), Corticosteroids, Biologics, TNF Inhibitors, Interleukin Inhibitors, and pain medications. The most important way of non-drug Ankylosing Spondylitis treatment which is very common is the education of patients, exercise physical therapy, good posture practices, and other options such as applying heat/cold to help relax muscles and reduce joint pain. In severe cases, posture correcting Ankylosing Spondylitis surgery may also be an option. Depending on the type of spondyloarthritis, there may be some variation in treatment. For example, in psoriatic arthritis, both the skin component and joint component must be treated. In enteropathic arthritis (spondylitis/arthritis associated with inflammatory bowel disease), medications may need to be adjusted so the gastrointestinal component of the disease is also treated and not exacerbated.
Non-steroidal Anti-Inflammatory Drugs (NSAIDs) such as Celecoxib (Celebrex), Diclofenac/Misoprostol (Arthrotec), Ibuprofen (Advil, Motrin, Nuprin), Indomethacin (Tivorbex), Meloxicam (Mobic), and Naproxen (Aleve, Anaprox, Naprosyn) are prescribed for symptomatic relief for Ankylosing Spondylitis. Disease-modifying Antirheumatic Drugs (DMARDs) work to limit tissue damage from inflammation such as Methotrexate (Otrexup, Rasuvo, Rheumatrex, Trexall), Sulfasalazine (Azulfidine) in Ankylosing Spondylitis treatment. FDA-approved biologics such as TNF inhibitors – Adalimumab (Humira), Certolizumab pegol (Cimzia), Etanercept (Enbrel), Golimumab (Simponi), Infliximab (Remicade), and other Interleukin inhibitors like Ixekizumab (Taltz), Secukinumab (Cosentyx) are recommended for Ankylosing Spondylitis treatment.
Emerging Drugs in Ankylosing Spondylitis Treatment Pipeline
Ankylosing Spondylitis pipeline possesses potential drugs as monotherapies as well as combination therapies. The expected launch of new drugs for Ankylosing Spondylitis are expected to create a significant shift in the Ankylosing Spondylitis treatment market in the coming years. Key players such as UCB Biopharma, Celgene and Inmagene Biopharmaceuticals, and many more are investigating their candidates for Ankylosing Spondylitis aiming to reduce the disease burden. All the upcoming Ankylosing Spondylitis therapies are either for prevention or treatment.
There are many Ankylosing Spondylitis pipeline drugs in different phases of development under clinical trials, which include –
Bimekizumab (UCB4940) is the first humanized monoclonal IgG1 antibody being developed by UCB Pharma. This therapy potently and selectively neutralizes IL-17A and IL-17F. These two key proinflammatory cytokines share similar biological functions and structural homology. IL-17A and IL-17F are the most closely related members of the IL-17 family of cytokines. They are both co-expressed at sites of inflammation and have overlapping proinflammatory functions. Both IL-17A and IL-17F can independently cooperate with other inflammatory mediators to drive chronic inflammation and tissue destruction. This therapeutic candidate is in the Phase III stage of development to treat Ankylosing Spondylitis patients. The company is using the subcutaneous route of administration.
Have You Read About the Growth Prospects of The Rapidly Evolving Ankylosing Spondylitis Market? Get Rich Insights Through Our Ankylosing Spondylitis Newsletter
CC-99677 is a novel, oral, selective MK2 inhibitor that is currently being investigated by Celgene. This therapy is considered to be a sustainable multi-cytokine inhibitor for Ankylosing Spondylitis treatment and other inflammatory diseases. The mitogen-activated protein kinase-activated protein kinase-2 (MK2) pathway is activated downstream of p38, and activation of MK2 increases the stability and translation of mRNA of proinflammatory factors (e.g., TNF-α, IL-17, IL-6). CC-88977 is in the Phase II stage of development for Ankylosing Spondylitis treatment.
Izokibep (ABY- 035) is a bi-specific fusion protein that targets the subunits of IL-17A and albumin. It is specifically designed by Inmagene Biopharmaceuticals to create a very small protein drug (18 kDa) with a very high apparent affinity to IL-17A. This therapeutic candidate is in the Phase II stage of development to treat Ankylosing Spondylitis patients. It has antibody-like half-life due to the strong binding affinity to serum albumin. The drug is manufactured in an inexpensive E. coli system which leads to notable lower manufacturing cost/dose.
QX 002N is currently being developed by Qyuns Therapeutics that comes under the classification of IL-17 inhibitors. The drug is in Phase I stage of clinical development for Ankylosing Spondylitis treatment. The company is using the subcutaneous route of administration.
KIN-1901 is under investigation by Kinevant Sciences as a Granulocyte macrophage colony stimulating factor antagonist for Ankylosing Spondylitis treatment. The drug is in Phase I stage of clinical development with a subcutaneous route of administration. The study will be an ascending single and multiple-dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of subcutaneous KIN-1901 in healthy subjects and Ankylosing Spondylitis subjects.
Unmet Needs in Ankylosing Spondylitis Treatment Domain
The presence of several unmet needs in the Ankylosing Spondylitis treatment landscape include
- Early and correct Ankylosing Spondylitis diagnosis which is crucial for successful therapy, but both under-diagnosed, with a gap between first symptoms and diagnosis of many years, and overdiagnosis are common.
- Understanding the causes/relationship of extra-articular disease, including bowel and eye disease to joint disease, remains an unmet need.
- There is also a need for more reliable biomarkers for ankylosing spondylitis, like those currently available that lack sensitivity and specificity.
Way Ahead
In the upcoming years, several new products including anti-IL17A, JAK inhibitors, and biologics are expected to get launched in the Ankylosing Spondylitis treatment market which means that the market might become increasingly competitive, while on other hand the high cost of therapies for the treatment of Ankylosing spondylitis is a major factor restraining the growth of the global Ankylosing Spondylitis biological drug market. Biosimilars’ entry in the market will offer a reduction in healthcare costs, and improve access to more biologics for Ankylosing Spondylitis. Also, the rising awareness of Ankylosing Spondylitis and the vast availability of treatment therapeutics will help in reducing the symptoms, ease inflammation, and possibly prevent permanent joint damage.

Frequently Asked Question (FAQ)
Ankylosing Spondylitis is a form of arthritis that primarily affects the spine. It causes severe inflammation of the spinal joints (vertebrae) that can lead to chronic pain and discomfort.
Yes, Ankylosing Spondylitis is a potentially disabling illness, as Ankylosing Spondylitis is an incurable, lifelong disease. Treatments and prescription medications can help patients reduce the effect of their symptoms, but the disease can be disabling.
Ankylosing Spondylitis is both an autoimmune type of arthritis and chronic inflammatory disease.
There is no specific cause of Ankylosing Spondylitis as genetics plays an important role in ankylosing spondylitis occurrence, over 90% of patients with this condition have a particular genetic marker in their white blood cells, called HLA-B27.
Key pharmaceuticals companies that are proactively involved in producing Ankylosing Spondylitis drugs in the coming years include names such as Pfizer, UCB Biopharma, Fresenius Kabi, Shanghai Henlius Biotech, Fujifilm Kyowa Kirin Biologics, Hetero Biopharma, Bio-Thera Solutions, Amgen, CinnaGen, Zydus cadilla, Cadila Pharmaceuticals, Sandoz, Innovent Biologics, Fresenius Kabi, Torrent Pharmaceuticals, Cipla, Emcure Pharmaceuticals, Biogen, and many more.




