Antibody-Drug Conjugate: The Smart Biological Bomb
Nov 30, 2022
Antibody-drug conjugate (ADCs) is a new emerging class of highly potent pharmaceutical drugs, which is a great combination of chemotherapy and immunotherapy. Over the past couple of decades, antibody-drug conjugates have revolutionized the field of cancer chemotherapy. ADCs are composed of 3 parts: an antibody drug, a cytotoxic payload, and a chemical linker protein to hold the 2 parts together.
ADCs payloads can be classified into two major types: (i) tubulin inhibitors inhibit tubulin polymerization and trigger cell cycle arrest in the G2/M phase and subsequent cell apoptosis; these inhibitors include monomethyl auristatin E (MMAE), monomethyl auristatin F (MMAF), and a derivative of maytansine 1 (DM1), (ii) DNA-damaging agents bind the minor groove in DNA, leading to cell death via DNA cleavage, DNA alkylation or interrupted DNA replication – these agents include calicheamicin, SN-38, DXd, and PBD. Other small-molecule payloads, such as α‐amanitin (a selective RNA polymerase II inhibitor), are also under investigation.
Based on the mechanism of payload release, linkers can be categorized into cleavable or non-cleavable linkers. Cleavable linkers are designed to conditionally respond to the TME or intracellular environments, such as low pH, proteolysis, and high-glutathione concentrations. On the other hand, noncleavable linkers rely on complete lysosomal degradation of the antibody for payload release. Noncleavable linkers are more stable in circulating blood than cleavable linkers, but they are not able to kill neighboring cancer cells by bystander effect, due to the lack of cell permeability related to the charged amino acid appendage. Cleavable linkers enhance the bystander effect and can distinguish between the circulatory and target cells conditions, making them the preferred choice to treat the majority of cancer types.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Can Cannabinoids be an Effective Medicinal Substance?
- Inozyme Raises $67M; Shanghai Miracogen partners with Synaffix; EdiGENE raises $15M; Antengene an...
- EU approval for Libtayo and Dovato; Boehringer expands NASH pipeline
- PD-1 and PD-L1 inhibitors – Competitive Landscape, Pipeline and Market Analysis, 2015
- First oral GLP-1 treatment approved for type2 Diabetes
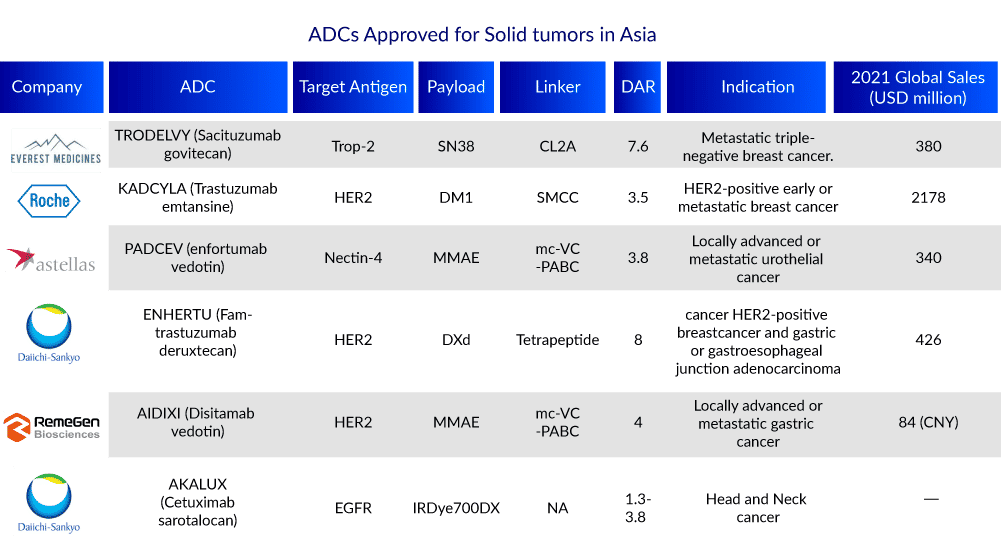
Drug-to-antibody ratio (DAR) is the average number of drugs conjugated to the antibodies, which is an important attribute of Antibody Drug Conjugate. The DAR value affects the efficacy of the drug, as low drug loading reduces the potency, while high drug loading can negatively affect pharmacokinetics (PK) and toxicity.
With the advancement in gene engineering technology, the ADC’s generation has evolved from First to third-generation ADCs. Third-generation ADCs are replacing the second-generation ADCs as in this generation, a fully human antibody is used instead of a chimeric antibody.
Antibody Drug Conjugate drugs are available earlier in the US, as compared to the Asian countries with up to 12 drugs on the market and sales well ahead of other regions, including Mylotarg(2010), Adcetris(2011), Kadcyla(2013), Besponsa(2017), Lumoxiti (2018), Polivy(2019), Padcev(2019), Enhertu(2019), Trodelvy(2020), Blenrep(2020) Zynlonta(2021) and Tridak(2021).
ACDs are slowly making their way to Asian countries for example Japan approved seven Antibody Drug Conjugate including Mylotarg (2005), Adcetris(2014), Kadcyla(2014), Besponsa (2018), Enhertu(2020), Akalux (2020), Padcev(2021), and Enhertu (under review).
The Chinese market has seen a boom in ADCs drugs. In 2020, Adcetris and Kadcyla, two of the world’s first Antibody Drug Conjugate drugs on the market, were approved for launch in China. In 2021, RemeGen’s Disitamab vedotin (Aidixi) and inotuzumab ozogamicin (Besponsa) were approved for marketing. In June 2022, the NMPA approved Trodelvy, also the regulatory approval of Enhertu is under review in China too. Many Chinese companies namely Hengrui, Miracogen, RemeGen, DAC Biotech, and many others, are developing Antibody Drug Conjugate.
In June 2022, the Health Sciences Authority of Singapore approved PADCEV (enfortumab vedotin) as the first antibody-drug conjugate approved in Singapore for locally advanced or metastatic urothelial cancer also Trodelvy, and Enhertu are approved in Singapore.
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The pharmaceutical industry is witnessing a dynamic shift from conventional technologies to newer and more robust approaches for the development of such complex biomolecules, with approximately 50 ADCs being investigated in more than 150 clinical trials. As the technology advances even further, personalized Antibody Drug Conjugate may be a possibility in the future and there may be the most appropriate antibody, linker, and payload based on the patient’s tumor antigens and other characteristics. Generating personalized ADCs within an acceptable timeframe and without prohibitively high costs will be a challenge. Wherever chemotherapy is currently used, ADCs could serve as the replacements of the future, and with personalized ADCs, the opportunities are enormous.

Downloads
Article in PDF
Recent Articles
- “Endometrial Cancer – Pipeline Insights, 2015”- A DelveInsight’s Report
- Evaluating the Key Trends and Developments in the Global Next-Generation Sequencing Market
- Gold Nanoparticles May Soon Treat Prostate Cancer
- Top 10 Expected Oncology Drug Launches in 2023
- ALLO-647 Integration in Lymphodepletion: Paving the Way for Enduring Responses and Safe CAR T cel...




