Understanding Immunohistochemistry: A Key Tool in Biomedical Research and Market Insights
Jul 30, 2025
Table of Contents
Immunohistochemistry has become an essential tool in both clinical and research settings, bridging the gap between molecular biology and histopathology. By combining immunology and histological techniques, it enables scientists and healthcare professionals to uncover intricate biological patterns and diagnose complex conditions with greater accuracy. Its applications span from understanding disease progression to identifying therapeutic targets, making it a cornerstone in modern pathology.
What is Immunohistochemistry?
Immunohistochemistry (IHC) is a powerful laboratory technique used to detect specific proteins, antigens, or other molecules in tissue samples by leveraging the specificity of antibodies. The immunohistochemistry test is widely employed in medical diagnostics and research to visualize the presence and distribution of target molecules within cells or tissues, aiding in the identification of diseases such as cancer, infectious diseases, and neurological disorders. By applying immunohistochemistry staining, researchers and clinicians can observe the localization of specific biomarkers under a microscope, providing critical insights into disease mechanisms, tissue architecture, and cellular processes. This technique has become indispensable in biomedical research and clinical diagnostics due to its precision and ability to provide detailed visual information about molecular interactions within biological samples.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- LensGen’s Juvene Intraocular Lens; Nevro announces clinical data publications; FDA Clearance to I...
- SIBIONICS’s Ground-breaking GS1 Continuous Glucose Monitoring System; Abbott’s HPV Test to Run on...
- Aerin Medical’s RhinAer Stylus; CE Mark for Haemonetics’s VASCADE Vascular Closure Device; J&...
- Revolutionizing Pain Management: The Growth in Innovative Devices and Rise of Market
- Artificial Intelligence in Clinical Trials: Transforming Drug Development Efficiency and Precision
The Science Behind Immunohistochemistry
The immunohistochemistry test operates on the principle of antigen-antibody interactions. The process begins with preparing thin tissue sections, typically fixed and embedded in paraffin, which are mounted onto slides. These sections are then treated with primary antibodies that specifically bind to the target antigen within the tissue. A secondary antibody, conjugated with a detectable marker such as a fluorescent dye or enzyme, is applied to amplify the signal. Immunohistochemistry staining techniques, such as chromogenic or fluorescent staining, are used to visualize the bound antibodies, making the target antigens visible under a microscope. The staining highlights the spatial distribution and intensity of the target molecules, enabling detailed analysis of their expression patterns. Advanced automation and machine learning have further enhanced the precision and reproducibility of immunohistochemistry staining, streamlining workflows in both research and diagnostic settings.
Key Components
The success of an immunohistochemistry test relies on several critical components:
- Antibodies: High-specificity primary and secondary antibodies are essential for accurate antigen detection.
- Reagents and Kits: These include buffers, blocking agents, and staining reagents that ensure optimal conditions for antigen-antibody binding and visualization.
- Instruments: Automated staining platforms and microscopes are used to perform and analyze immunohistochemistry staining with high throughput and consistency.
- Tissue Samples: Properly prepared and preserved tissue sections are crucial for reliable results. These components work together to make immunohistochemistry a versatile and robust tool for studying protein expression in various biological contexts, from cancer diagnostics to infectious disease research.
Applications in Research and Medicine
Immunohistochemistry (IHC) has a wide range of applications in both research and clinical settings, making it a cornerstone technique in understanding and diagnosing various diseases. By enabling the visualization of specific proteins and antigens in tissue samples, IHC provides critical insights into disease mechanisms and aids in the development of targeted treatments. Below are some of the key applications of immunohistochemistry in research and medicine.
Comparison of H&E Stain vs. IHC Stain in Breast Tissue
| Feature | H&E Staining (Normal Tissue) | IHC Staining (HER2+ Breast Cancer) |
| Purpose | Highlights general tissue architecture and cell morphology | Detects specific proteins/antigens (e.g., HER2 receptor) |
| Staining Mechanism | Uses Hematoxylin (nuclei, blue) and Eosin (cytoplasm, pink) | Uses antibodies tagged with chromogen to detect target antigens |
| Appearance Under Microscope | Uniform pink/blue hues, clear cellular detail | Brown/red membrane staining where HER2 is overexpressed |
| Molecular Detail | No specific protein identification | Visualizes overexpression/localization of HER2 protein |
| Diagnostic Use | Initial screening and structural evaluation | Confirms molecular subtype, guides targeted therapy (e.g., trastuzumab) |
| Tissue Example | Normal breast epithelial tissue | HER2-positive breast carcinoma tissue |
Cancer Diagnostics
The immunohistochemistry test is extensively used in cancer diagnostics to identify specific tumor antigens that are either newly expressed or overexpressed in cancerous tissues. Immunohistochemistry staining allows pathologists to detect biomarkers such as PD-L1, HER2, or hormone receptors, which are critical for determining cancer type, stage, and treatment options. For instance, the use of IHC in detecting PD-L1 expression helps identify patients with cancers like triple-negative breast cancer who may benefit from immunotherapies such as pembrolizumab. With approximately 19.3 million new cancer cases reported globally in 2020, according to the GLOBOCAN directory, the demand for IHC in cancer diagnostics continues to grow, supporting personalized medicine and improving patient outcomes.
Neuroscience Research
In neuroscience, immunohistochemistry staining is a vital tool for studying the cellular and molecular mechanisms underlying neurological disorders. IHC is used to map the expression of proteins such as tau, amyloid-beta, or neuronal markers in brain tissue, aiding in the study of conditions like Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. By visualizing the distribution of these proteins, researchers can better understand disease progression and identify potential therapeutic targets. The precision of the immunohistochemistry test allows for detailed analysis of neuronal structures and pathological changes, contributing significantly to advancements in neuroscience research.
Infectious Disease Studies
Immunohistochemistry plays a crucial role in the detection and identification of infectious agents, including viruses, bacteria, fungi, and parasites. The immunohistochemistry test uses specific antibodies to detect antigens associated with pathogens, such as the herpes simplex virus (HSV) or hepatitis B virus, in tissue samples. According to the World Health Organization, approximately 3.7 billion people under 50 have HSV-1, and 296 million people were living with chronic hepatitis B in 2019, highlighting the global burden of infectious diseases. Immunohistochemistry staining enables researchers and clinicians to confirm the presence of these pathogens in tissues, facilitating accurate diagnosis and informing treatment strategies. The technique has also been adapted for studying emerging infectious diseases, such as COVID-19, with companies like Bio SB developing IHC products to detect SARS-CoV-2 antigens in tissue biopsies.
Advantages and Limitations
Immunohistochemistry (IHC) is a highly valued technique in both research and clinical diagnostics due to its unique strengths, but it also comes with certain challenges that must be considered. Understanding the benefits and limitations of the immunohistochemistry test, including its comparison to related techniques like immunofluorescence, is essential for its effective application.
Benefits of Immunohistochemistry
The immunohistochemistry test offers several key advantages that make it a preferred method in biomedical research and diagnostics:
- High Specificity and Sensitivity: Immunohistochemistry staining allows for the precise detection of specific antigens in tissue samples, providing detailed insights into protein expression and localization.
- Versatility in Applications: IHC is used across diverse fields, from cancer diagnostics to neuroscience and infectious disease studies, making it a versatile tool for both research and clinical settings.
- Compatibility with Fixed Tissues: Unlike some other techniques, IHC can be performed on formalin-fixed, paraffin-embedded (FFPE) tissues, which are widely available in clinical archives, enabling retrospective studies.
- Visual Clarity: The use of chromogenic or fluorescent markers in immunohistochemistry staining provides clear, high-resolution visualization of target molecules under a microscope, aiding in accurate interpretation.
- Automation and Scalability: Advances in automated staining platforms and reagents have improved the reproducibility and throughput of IHC, making it suitable for large-scale studies and diagnostics. When comparing immunohistochemistry vs immunofluorescence, IHC has an edge in routine diagnostics due to its compatibility with standard light microscopy and the stability of chromogenic stains, which do not require specialized fluorescence microscopes and are less prone to fading over time.
Challenges and Considerations
Despite its advantages, the immunohistochemistry test has some limitations that can impact its application:
- Risk of False-Positive Results: Non-specific binding of antibodies or improper tissue preparation can lead to inaccurate results, requiring careful optimization and validation of protocols.
- High Cost of Instruments and Reagents: The advanced equipment and high-quality antibodies required for immunohistochemistry staining can be expensive, potentially limiting access in resource-constrained settings.
- Technical Complexity: IHC requires skilled personnel to perform tissue preparation, antibody selection, and staining, as well as to interpret results accurately.
- Limited Multiplexing: Compared to immunofluorescence, which allows for simultaneous detection of multiple antigens using different fluorescent markers, traditional immunohistochemistry staining is often limited to detecting one or two targets at a time due to chromogenic constraints. In the context of immunohistochemistry vs immunofluorescence, immunofluorescence offers superior multiplexing capabilities and higher resolution for co-localization studies, but requires specialized equipment and is less suited for long-term sample storage. These trade-offs must be considered when choosing between the two techniques for specific research or diagnostic needs.
Recent Advances and Innovations
The field of immunohistochemistry (IHC) has seen significant advancements in recent years, driven by technological innovations and the integration of artificial intelligence (AI). These developments have enhanced the precision, efficiency, and scope of immunohistochemistry tests, making them even more valuable in research and diagnostics. Below are some notable recent innovations in IHC, listed in descending order by date.
In April 2025, Roche announced that the U.S. Food and Drug Administration (FDA) granted Breakthrough Device Designation for the VENTANA TROP2 (EPR20043) RxDx Device, the first computational pathology companion diagnostic (CDx) to receive this designation. This immunohistochemistry assay, combined with a digital pathology algorithm, enhances tissue pathology by using AI to improve TROP2 scoring, aiding clinicians in selecting targeted therapies for cancer patients and improving diagnostic accuracy.
In March 2025, a retrospective study published on March 31, 2025, highlighted an AI model capable of accurately classifying prostate biopsy H&E images, reducing the need for immunohistochemistry tests by 20–44% without compromising diagnostic reliability (arXiv). This advancement optimizes workflows in prostate cancer diagnostics, cutting costs and minimizing the dependency on immunohistochemistry staining for certain cases, while maintaining high diagnostic standards.
In March 2025, discussions on platforms like Reddit highlighted increased FDA scrutiny over laboratory-developed tests (LDTs), including IHC protocols, particularly for decalcified tissues (arXiv, Reddit). This regulatory focus emphasizes the need for thorough validation of immunohistochemistry tests to ensure reliability and compliance, potentially reshaping lab practices and enhancing the standardization of immunohistochemistry staining procedures.
In April 2021, Roche received FDA approval for the VENTANA MMR RxDx Panel, the first immunohistochemistry predictive test in endometrial cancer for treatment with the anti-PD1 immunotherapy JEMPERLI (dostarlimab-gxly). This approval marked a significant step in expanding IHC applications for personalized cancer treatment.
In March 2021, Roche launched the DISCOVERY Green HRP kit, expanding the multiplexing capabilities of IHC. This kit allows for the simultaneous detection of multiple biomarkers in tissue-based research, offering distinct chromogenic colors that enhance visualization. Such advancements improve the efficiency of immunohistochemistry tests and support complex studies requiring detailed protein profiling.
These innovations, coupled with ongoing advancements in automation and machine learning, are transforming immunohistochemistry into a more precise, scalable, and cost-effective tool, further solidifying its role in tissue pathology and clinical diagnostics.
Market Insights for Immunohistochemistry
The immunohistochemistry market has experienced significant growth in recent years, driven by increasing demand for precise diagnostic tools and advancements in technology. Below is an in-depth look at the key aspects of the immunohistochemistry market, including its size, segmentation, key players, and regional dynamics.
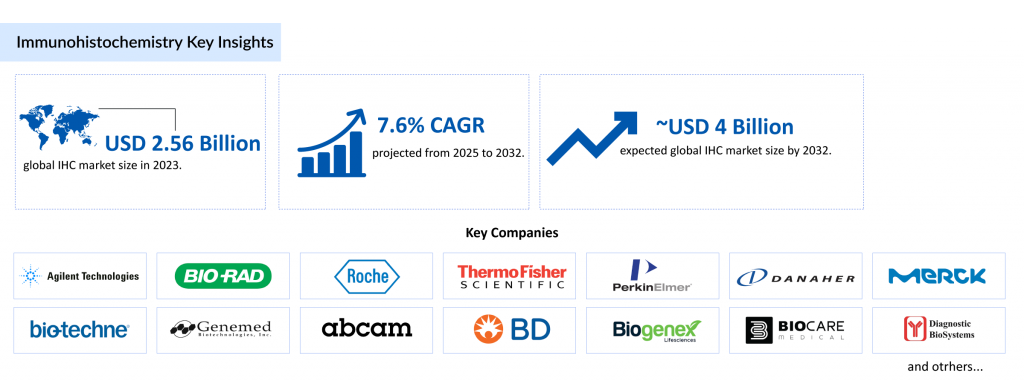
Market Size and Growth
The global immunohistochemistry market was valued at USD 2.56 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 7.64% from 2025 to 2032, reaching USD 3.98 billion by 2032. This growth is primarily driven by the rising prevalence of chronic and infectious diseases, such as cancer, hepatitis B, and herpes simplex virus, which increase the demand for immunohistochemistry tests. For instance, the World Health Organization reported in 2020 that approximately 3.7 billion people under age 50 have HSV-1, and 296 million people were living with chronic hepatitis B in 2019, underscoring the global need for IHC in infectious disease diagnostics. Additionally, the increasing burden of cancer, with 19.3 million new cases reported globally in 2020 according to the GLOBOCAN directory, further fuels the immunohistochemistry market. The growing adoption of automation, machine learning, and advanced IHC products also contributes to this robust market expansion.
Market Segmentation
The immunohistochemistry market is segmented by product type, application, end-user, and geography:
- Product Type: The market includes instruments, reagents & kits, and services. The reagents & kits segment, particularly the immunohistochemistry antibody market, holds a significant share due to the wide availability of specialized antibodies and kits that reduce errors in tissue staining. For example, Leica Biosystems (Danaher Corporation) received FDA clearance in March 2020 for its BOND Ready-to-Use Primary Antibody Progesterone Receptor, boosting the immunohistochemistry antibody market.
- Application: IHC is used in diagnostics, research, and other applications. Diagnostics, especially cancer diagnostics, dominates due to the critical role of immunohistochemistry staining in identifying tumor-specific antigens like PD-L1 and HER2.
- End-User: Key end-users include hospitals & diagnostic laboratories, academic & research institutes, and others. Hospitals and diagnostic laboratories lead due to the high volume of IHC tests performed for clinical purposes.
- Geography: The market is divided into North America, Europe, Asia-Pacific, and the Rest of the World, with North America holding the largest share, driven by high cancer and infectious disease prevalence and advanced healthcare infrastructure.
Key Players and Developments
The immunohistochemistry market is highly competitive, with key players such as Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., F. Hoffmann-La Roche Ltd, Thermo Fisher Scientific, and Danaher Corporation driving innovation. Recent developments include Roche’s VENTANA TROP2 RxDx receiving FDA Breakthrough Device Designation in April 2025, marking a milestone in AI-integrated immunohistochemistry assays.
Additionally, companies like Bio SB have expanded the immunohistochemistry antibody market by developing products for COVID-19 research, such as antibodies for SARS-CoV-2 detection. These advancements, coupled with strategic approvals and product launches, are strengthening the market position of these players and enhancing the capabilities of immunohistochemistry tests.
Regional Analysis
North America dominates the global immunohistochemistry market, driven by the high incidence of cancer (2.28 million new cases in the US in 2020, per GLOBOCAN) and infectious diseases like hepatitis B and C (4,783 and 12,477 cases in Canada in 2018, respectively). The region benefits from the presence of key market players, robust research and development activities, and the adoption of advanced IHC technologies, such as automated staining platforms. For instance, the FDA approval of PD-L1 IHC 22C3 pharmDx in November 2020 as a companion diagnostic for pembrolizumab highlights North America’s leadership in integrating IHC into personalized medicine. Europe and Asia-Pacific are also significant markets, with growing research activities and increasing healthcare investments, while the Rest of the World is expected to see gradual growth due to improving diagnostic infrastructure.
The immunohistochemistry market, particularly the immunohistochemistry antibody market, continues to evolve with technological advancements and increasing disease prevalence, positioning IHC as a critical tool in modern diagnostics and research.
Conclusion
Immunohistochemistry (IHC) remains a cornerstone of biomedical research and clinical diagnostics, offering unparalleled precision in detecting and visualizing specific proteins and antigens in tissue samples. The immunohistochemistry test has proven its value across diverse applications, from cancer diagnostics and neuroscience research to infectious disease studies, driven by its high specificity and compatibility with fixed tissues. Despite challenges such as the risk of false-positive results and high costs, advancements like AI-integrated assays, such as Roche’s VENTANA TROP2 RxDx, and multiplexing innovations like the DISCOVERY Green HRP kit are enhancing the efficiency and accuracy of immunohistochemistry staining. The immunohistochemistry market is fueled by rising disease prevalence and technological progress, with the immunohistochemistry antibody market playing a pivotal role. As regulatory scrutiny increases and automation continues to streamline workflows, IHC is poised to further revolutionize personalized medicine and research, solidifying its critical role in advancing healthcare outcomes.

Downloads
Article in PDF
Recent Articles
- Accutar’s Phase I clinical trial for AC0176; Bio-Thera’s cancer drug, BAT6005; Nykode...
- Novel Genetics Biomarker found by a Group of Researchers May Offer Novel Treatment Approach for C...
- Pioneering Progress: ZYNLONTA and Rituximab Combo Delivers Striking 96% Overall Response in Relap...
- FDA Grants 510(k) Clearance to Nerveblox AI; Labcorp Introduces the First FDA-Cleared Alzheimer’s...
- Juvenescence nets USD 100M; Sarepta DMD drug faces rejection



