Atopic Dermatitis Treatment: Rich Portfolio of Therapeutic Options Shaping the Future
May 20, 2024
Atopic dermatitis is common worldwide, and people of all ages, from newborns to adults 65 and older, live with this condition. The World Health Organization (WHO) Global Burden of Diseases initiative estimates more than 230 million people globally have atopic dermatitis and that skin diseases are the fourth leading cause of non-fatal disability worldwide.
As per the DelveInsight assessment, the total prevalent cases of atopic dermatitis in the 7MM comprised ~72 million cases in 2022 and are projected to increase by 2032. As per the estimates, the US contributed to the largest prevalent population of atopic dermatitis, acquiring ~45% of the 7MM in 2022. Whereas Japan accounted for around 13%, and the United Kingdom accounted for around 10% of the total population share, respectively, in 2022.
Atopic dermatitis doesn’t have a cure, but it can be well controlled through available treatments. The atopic dermatitis treatment approach depends on the type and severity of the condition. In the US, Europe, and Japan, topical atopic dermatitis treatments like emollients, topical corticosteroids with antibiotics, and topical calcineurin inhibitors are commonly used, alongside systemic atopic dermatitis treatments such as immunosuppressants, corticosteroids, DUPIXENT (dupilumab), and phototherapy.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- VistaGen’s PH94B for Anxiety Disorder; Keytruda for Head and Neck Cancer Treatment; Bavarian Nord...
- Merck Wins FDA Approval for KEYTRUDA QLEX for Subcutaneous Use in Adults With Solid Tumors; Incyt...
- Immunocore’s Kimmtrak; Samsung Acquires Biogen’s Biosimilar Unit; Novavax’s COVID-19 Vaccin...
- Immutep’ First-Line Treatment Positive Outcomes; Pfizer’s Once-Daily Oral GLP-1 Agonist Danuglipr...
- Novo Nordisk A/S Announces that CagriSema Delivered Superior Outcomes in REIMAGINE 2; Sanofi’s Ve...
Drugs like OLUMIANT (baricitinib) have received approval in Europe and Japan, while CORECTIM (delgocitinib) ointment 0.5% is only approved in Japan, and EUCRISA (crisaborole) 2% ointment is approved in both the US and Europe. In managing atopic dermatitis, maintenance involves frequent application of emollients and daily bathing with soap-free cleansers. Topical corticosteroids (TCS) are the primary atopic dermatitis treatment for flare-ups, with pimecrolimus and tacrolimus being topical calcineurin inhibitors (TCIs) that can complement TCS therapy.

As per DelveInsight analysis, the atopic dermatitis market size is supposed to grow from USD 15.6 billion in 2022 at a CAGR of ~8% during the forecast period owing to the expected launch of novel emerging therapies, which shall fuel the growth of the atopic dermatitis treatment market during the forecast period, i.e., 2023–2032. As per the estimates, the United States accounted for the highest market size of atopic dermatitis in 7MM in 2022, in comparison to the other major markets i.e., EU5 countries (the United Kingdom, Germany, Italy, France, and Spain), and Japan.
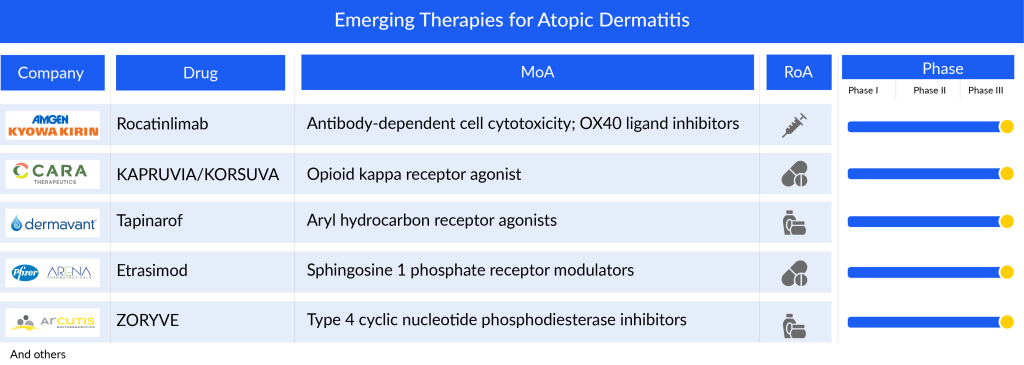
The current atopic dermatitis pipeline consists of a great deal of drugs. Roflumilast, Rocatinlimab, Tapinarof, KAPRUVIA/KORSUVA (difelikefalin), and some others are the most highlighted drugs of this indication. Ongoing research and current trials have the potential to change the atopic dermatitis treatment market.
The potential for effective atopic dermatitis treatment in the future is expected to grow with the addition of numerous drugs to the pipeline of new therapeutics. Currently, a variety of candidate drugs for the treatment of atopic dermatitis are being tested in various clinical trials, with some showing positive results. Any drug approved with increased safety and efficacy is expected to cause significant changes in the overall atopic dermatitis market.
Now, let’s examine the pipeline therapies under investigation for atopic dermatitis
Roflumilast: Arcutis Biotherapeutics
Phase III
The ZORYVE (roflumilast) cream, a topical medication, functions by inhibiting phosphodiesterase-4 (PDE4), an enzyme within cells that promotes the production of inflammatory substances while reducing anti-inflammatory substances. This mechanism makes it effective in treating various inflammatory conditions such as psoriasis, atopic dermatitis, and chronic obstructive pulmonary disease (COPD). PDE4 inhibitors are well-established in dermatology, with some approved by the FDA for systemic treatment of plaque psoriasis. Roflumilast cream, specifically formulated for once-daily use, is a potent and selective PDE4 inhibitor. Arcutis’ studies on atopic dermatitis have demonstrated its efficacy. In November 2023, the FDA accepted Arcutis’ application to expand the use of Roflumilast Cream 0.15% for treating atopic dermatitis in adults and children as young as 6 years old. This decision was supported by positive results from the Phase III trials INTEGUMENT-1 and INTEGUMENT-2, affirming its efficacy and safety.
Rocatinlimab: Amgen/Kyowa Kirin
Phase III
Rocatinlimab, an anti-OX40 human monoclonal antibody, functions by impeding and diminishing the population of OX40-expressing pathogenic T cells that instigate systemic and local inflammatory reactions. These T cells, identified for their OX40 expression, play a pivotal role in the pathophysiology of atopic dermatitis, as observed in patients’ lesions. The initial discovery of this antibody stemmed from a collaboration between Kyowa Kirin US Research and the La Jolla Institute for Immunology. Amgen and Kyowa Kirin have recently initiated the recruitment of participants for a comprehensive global Phase III program called the ROCKET program. This program aims to assess the safety and effectiveness of rocatinlimab in a diverse population of moderate-to-severe atopic dermatitis sufferers who bear a substantial disease burden. The company has indicated a projected approval timeframe for this atopic dermatitis drug around 2026/2027.
Tapinarof: Dermavant Sciences
Phase III
Tapinarof represents a new type of aryl hydrocarbon receptor agonist currently in the developmental phase. It’s formulated to be applied topically once a day, devoid of steroids, and designed for a pleasing cosmetic experience. Dermavant is spearheading its development specifically for addressing psoriasis and atopic dermatitis. Notably, tapinarof has undergone extensive evaluation, involving more than 1,400 individuals across 12 clinical trials. In the United States, VTAMA cream has gained approval for treating plaque psoriasis in adults. Presently, Dermavant is conducting two Phase III trials (NCT05014568, NCT05032859) targeting both children and adults with atopic dermatitis. Additionally, there’s a Phase III long-term extension trial (NCT05142774) for patients who have finished treatment in either of two pivotal studies, all of which are ongoing in the US. In March 2023, encouraging top-line results from the Phase III ADORING 2 trial were revealed, demonstrating successful achievement of all primary and secondary objectives.
KAPRUVIA/KORSUVA (difelikefalin): Cara Therapeutics
Phase III
KAPRUVIA/KORSUVA (difelikefalin) is a novel orally administered compound developed by Cara Therapeutics. It’s designed to specifically target kappa opioid receptors outside of the central nervous system (CNS), rather than mu receptors. This design includes unique chemical properties to limit its penetration into the CNS, focusing its action on kappa receptors in the peripheral nervous system. Unlike medications that activate mu receptors in the CNS, which can lead to various side effects including psychiatric issues, difelikefalin aims to avoid these by targeting kappa receptors. Cara Therapeutics has finished Phase II trials involving adult patients with atopic dermatitis and moderate to severe itching. Currently, it’s undergoing Phase III trials (NCT05387707; KIND-1) to assess difelikefalin’s effectiveness and safety as an additional therapy alongside topical corticosteroids for moderate-to-severe itching in adults with atopic dermatitis. The company anticipates releasing the top-line results of the KIND 1 trial by the first half of 2025.
Etrasimod: Pfizer/Arena Pharmaceuticals
Phase II/III
Etrasimod, developed by Pfizer, is an advanced oral medication taken once daily. It targets specific receptors known as sphingosine 1-phosphate (S1P) receptors, particularly 1, 4, and 5, with a high level of precision. This targeted approach aims to enhance its effectiveness and safety. Etrasimod is designed to exert both systemic and localized effects on particular types of immune cells, potentially offering treatment benefits for various immune-related inflammatory conditions such as ulcerative colitis, Crohn’s disease, eosinophilic esophagitis, atopic dermatitis, and alopecia areata. Pfizer has completed Phase II clinical trials (NCT04162769; ADVISE) for individuals with moderate-to-severe atopic dermatitis and is currently conducting Phase II/III trials (NCT05732454) in adults with moderate to severe atopic dermatitis who have previously tried other oral or injectable treatments.
BMX-010: BioMimetix
Phase II
BioMimetix is currently in the developmental stages of BMX-010, a diminutive compound known as redox-active metalloporphyrin (MnP). In 2023, the company commenced a Phase II clinical trial (NCT05491447) of BMX-010 ointment, aiming to assess its safety, clinical impacts, and how it interacts within the body in adults with atopic dermatitis. The primary conclusion of this trial is projected to occur around November 2024.
The expected launch of upcoming atopic dermatitis therapies and greater integration of early patient screening, medication in secondary care and other clinical settings, research on best methods for implementation, and an upsurge in awareness will eventually facilitate the development of effective treatment options. However, there are a few roadblocks regarding the timely diagnosis and treatment of these patients, for instance, the entry of generics due to the expiration of the patent protection and increasing healthcare expenses because the current atopic dermatitis treatment is lifelong. These factors often become a hindrance when adopting newer therapies.

Frequently Asked Questions
The atopic dermatitis pipeline features several promising therapies. Key candidates include: Rocatinlimab: An anti-OX40 monoclonal antibody targeting T-cell activation. Tapinarof: A topical aryl hydrocarbon receptor modulator with anti-inflammatory properties. Difelikefalin (KORSUVA): A kappa opioid receptor agonist under investigation for its anti-pruritic effects. Rezpegaldesleukin: A Treg cell-based therapy aiming to restore immune tolerance. These therapies are in various stages of clinical development, with some nearing regulatory approval.
ZORYVE is a topical cream that inhibits phosphodiesterase-4 (PDE4), an enzyme involved in inflammatory processes. By reducing PDE4 activity, ZORYVE helps decrease inflammation and itching associated with atopic dermatitis. It has been approved for use in adults and children aged six and older.
Janus kinase (JAK) inhibitors, such as upadacitinib and abrocitinib, are oral medications that target specific enzymes involved in the inflammatory process. These inhibitors have shown high short-term efficacy in treating moderate-to-severe atopic dermatitis. However, long-term safety and efficacy are still under evaluation.
Recent approvals include: Opzelura (ruxolitinib): A topical JAK1/2 inhibitor approved for adults and children aged 12 and older. ZORYVE (roflumilast): Approved for mild-to-moderate atopic dermatitis in individuals aged six and older. These approvals expand the available treatment options for patients with atopic dermatitis.
Rezpegaldesleukin is a biologic agent that acts as an interleukin-2 (IL-2) receptor agonist, stimulating regulatory T cells (Tregs). By enhancing Treg activity, it aims to restore immune tolerance and reduce inflammation in atopic dermatitis. Clinical trials have demonstrated its potential efficacy and safety profile.
Downloads
Article in PDF
Recent Articles
- Atopic dermatitis market and a Dupilumab dominance
- Immutep’ First-Line Treatment Positive Outcomes; Pfizer’s Once-Daily Oral GLP-1 Agonist Danuglipr...
- Atopic dermatitis market: Increasing prevalence and topical treatments
- Sanofi Strengthens Amlitelimab’s Position in Atopic Dermatitis; Corcept Announces Statistically S...
- DUPIXENT Receives First-Ever Biologic Approval for COPD: Adds Another Jewel in its Crown




