Peripheral Artery Disease Treatment: Role of Cell Therapies in Restoring Blood Circulation & Limb Function
Mar 31, 2023
Table of Contents
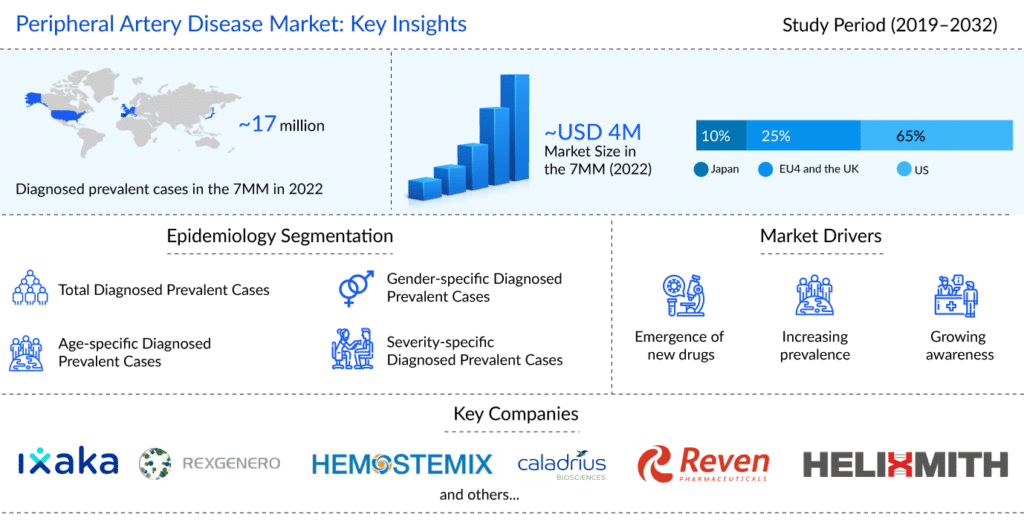
Peripheral artery disease (PAD) is one of the leading causes of cardiovascular morbidity, affecting 12-14% of the world’s population. Peripheral artery disease affects six times more people worldwide than HIV. 1 in 3 diabetics over age 50 suffer from peripheral artery disease. In addition, 1 in every 20 Americans over the age of 50 has peripheral artery disease. Approximately 150K lower-limb amputations in the U.S. can be attributed to peripheral artery disease yearly.
As per the latest published “Peripheral Artery Disease Epidemiology Forecast” report, the total prevalent cases of peripheral artery disease in the 7MM comprised of approximately 17 million cases in 2022 and are projected to increase during the forecasted period (2023–2032). According to DelveInsight estimates, the US accounted for the highest diagnosed prevalent cases of peripheral artery disease, followed by EU4, the UK, and Japan in 2022.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Mindray’s TE Air Handheld Ultrasound System; Getinge’s iCast™ Covered Stent System; Clinic...
- First Patient Enrolled in Kaizen Clinical Study in Japan; Koneksa’s At-Home Mobile Spirometry Cli...
- Asahi Intecc Partners with Penumbra; Prosoma and EVERSANA Announce Commercialisation Partnership;...
- Centinel Spine’s Prodisc C Match-the-Disc Cervical TDR System; Reflow Medical’s DEEPER OUS Trial;...
- GenScript Enters Distribution Agreement with EUROIMMUN; Biomerics’s New Facility in Galway (Irela...
The primary etiology of the disease is atherosclerosis, characterized by the deposition of lipids in the arteries and the blockage of normal arterial flow. Patients experience intermittent claudication (usually calf or thigh pain) as the disease progresses, especially during exertion. The most severe form of peripheral arterial disease is critical limb ischemia (CLI), marked by chronic ischemic pain with ulcer or gangrene.
Curative peripheral artery disease treatment options are limited, and disease management includes symptomatic peripheral artery disease treatments for pain and wound healing and the prevention of disease worsening into a stroke or other cardiovascular complications. Lifestyle changes are essential to control risk factors such as hypertension and diabetes. The therapeutic standard of care consists of anti-platelet medications, anti-hypertensive, and lipid-lowering agents, which are moderately effective in patients with mild to moderate peripheral artery disease.
For patients with severe claudication or those with critical limb ischemia, endovascular or surgical revascularization may be needed. Various revascularization techniques are available to restore in-line flow to the limbs, including endovascular, open, and hybrid procedures. The clinical choice of intervention to be employed is based on the severity of the disease, chances of success, and possible outcomes.
No option critical limb ischemia patients are those that are ineligible for revascularization due to failure of previous interventions, various comorbidities, attendant risk of surgery, and associated poor outcomes. These patients have a poor prognosis and are at risk of limb amputation due to severe ischemia. A monumental unmet need exists for these patients for the treatment of ulcers or ischemia to help resurrect arterial supply to the limbs reversing the condition.
Current Peripheral Artery Disease Treatment Scenario
The goals of peripheral artery disease treatment vary according to the severity of the disease. All patients with symptomatic or asymptomatic PAD have a primary concern: lowering the risk of cardiovascular morbidity and mortality. Improving functional status is an additional goal for patients with IC. Finally, preventing leg amputation, restoring mobility, and lowering mortality are critical for CLI patients. Different peripheral artery disease treatment options are available depending on the population and the goal. Tobacco use, diabetes, low-density lipoprotein levels, and hypertension are all considered risk factors that should be managed.
Exercise training, medical therapy, and revascularization are the three primary peripheral artery disease treatment options for improving functional status in patients with intermittent claudication. Several drugs have been approved for the treatment of IC, including naftidrofuryl oxalate, a 5-hydroxytryptamine type 2 antagonist; cilostazol, a phosphodiesterase III inhibitor; pentoxifylline, a vasodilator; and inositol nicotinate, a vasodilator. All have randomized controlled evidence of some improvement in walking distance.
Several regenerative therapies, including angiogenic recombinant proteins, gene therapy, cell-based therapies (including stem or progenitor cells), and chemokines, have been tested in patients with PAD and nonrevascularizable CLI. Collategene (HGF plasmid) is the only gene therapy approved in Japan. The company also plans to enter the US peripheral artery disease treatment market. Aside from that, many cell therapies are in the works. Many cell therapies are being developed to target the population that is not suitable for revascularization or had poor results after revascularization.
Novel Cell Therapies for Peripheral Artery Disease Treatment
Cell therapies open new doors for advanced peripheral artery disease treatment and are being discussed comprehensively as novel regenerative medicine. Upon administration into the patient’s limb, cell therapy targets activating the angiogenesis of neovessels through the release of growth factors and restoring the perfusion in the limbs. The improved blood supply reduces ischemic episodes and may cause the activation of regenerative pathways.
REX-001 (NCT03174522) by Ixaka Ltd. is one such autologous multi-cell therapy in Phase III, aimed to treat ulcers of patients with severity category Rutherford 5. With positive interim results, the therapy showed healing in over 70% of chronic limb-threatening ischemic patients receiving it.
A similar autologous intramuscular cell therapy, ACP-01 (NCT02551679), is being developed by Hemostemix, which can treat vascular disease. This emerging therapy for peripheral artery disease treatment has also shown promising results in its primary outcome of the Phase II trial, with comparatively fewer treated patients requiring amputation than those taking a placebo.
SAKIGAKE designated Honedra by Lisata Therapeutics, is another regenerative therapy in the mid-development stage. It is CD34+ cell therapy intended for intramuscular administration, 20 injections in the target limb in a single treatment. The CD34+ cell was discovered due to a deliberate search for a stem cell capable of stimulating blood vessel development and repair. The preliminary clinical responses are consistent with a positive therapeutic effect and safety profile, as well as previous clinical trials of CD34+ cell therapy in Japan and elsewhere. It is currently in Phase II clinical trial for peripheral artery disease treatment.
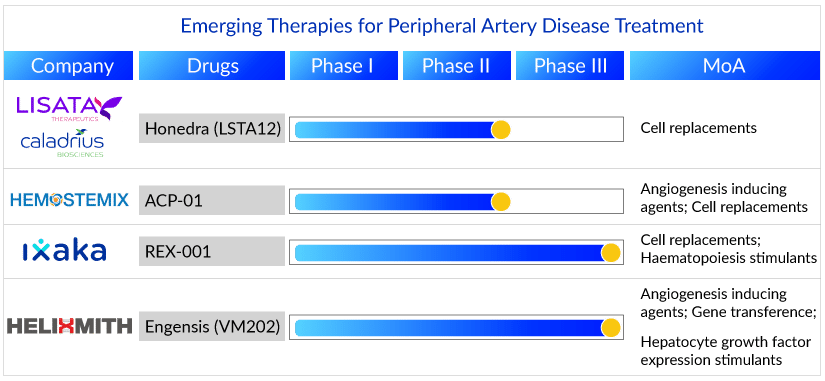
The implications of the results presented for these therapies provide an encouraging aspect to treating severe PAD. REX-001 is anticipated to launch in the US in 2024. Delveinsights’ forecast model estimates that REX-001 would capture a peripheral artery disease treatment market of around USD 3 million in the US in the launch year and may attain its peak sales in around seven years. ACP-01 (NCT02551679) is expected to launch soon after in 2025 and add about USD 247 million to the US peripheral artery disease treatment market by 2032.
As per DelveInsight analysis, the total peripheral artery disease market size in the 7MM was approximately USD 4 billion in 2022 and is projected to increase at a paltry CAGR of 4% during the forecast period (2023–2032). Moreover, cell therapies are estimated to significantly advance the peripheral artery disease treatment market’s revenue potential, taking up more than 50% of the overall market by 2032.
Peripheral Artery Disease Treatment Market: Pricing and Market Access
While cell therapies represent an entirely new approach to peripheral artery disease treatment, they will come in at a premium cost. New levels of innovation in pricing and market access strategies will be pivotal to their success.
The challenges around pharmacoeconomics are surmountable. As per the analysis by Mycka J et al. (2022), most cell therapies for peripheral artery disease treatment in the US are reimbursed, whereas, in different European countries, around 50–80% of such therapies fall under insurance coverage. The potential players will require to prove significant unmet needs with the existing standard of care and exhibit cost-effectiveness of their therapy with robust evidence.
KOL and Analyst Views on Changing Peripheral Artery Disease Market Dynamics
Progress in the clinical trials of cell therapies for peripheral artery disease treatment are being observed very optimistically by experts in the field. It is believed that the treatment landscape of peripheral artery disease will likely change momentously. The benefits will be appreciated in the long term with the decline in amputation rates. Additionally, the success of regenerative therapies in other indications provides hope that cell therapies for peripheral artery disease treatment will be launched at a reasonable price.
As already discussed, although revascularization is the first line of peripheral artery disease treatment for patients with severe PAD, almost one-third of patients may not be eligible and may need to make do with conservative treatments or undergo amputation. For those who do undergo revascularization, medical comorbidities may affect their recovery from the operation, quality of life, or survival. Cell therapies for peripheral artery disease treatment may prove to be a ray of hope for these patients toward preventing limb amputation and healing ulcerated wounds.
Evolving Landscape of Peripheral Artery Disease Treatment Market
The dynamics of the peripheral artery disease treatment market is expected to change in the coming years. The rising prevalence of PAD due to an aging population, combined with the continued rise in diabetes prevalence, is expected to propel the peripheral artery disease treatment market in the future, creating opportunities for new pharma players. New technologies and advancements in existing therapies are expected to improve endovascular therapy outcomes during the forecast period.
Moreover, several regenerative therapies, including angiogenic recombinant proteins, gene therapy, cell-based therapies (including stem or progenitor cells), and chemokines, have been tested in patients with PAD and nonrevascularizable CLI. Collategene (HGF plasmid) is the only gene therapy approved in Japan. The company also plans to enter the US peripheral artery disease market. Aside from that, many cell therapies are in development. Many cell therapies are being developed to target populations that are not candidates for revascularization or had poor outcomes after revascularization. Thus, the abovementioned factors will boost the peripheral artery disease treatment market in the coming years.
FAQs
Peripheral artery disease (PAD) is a common manifestation of lower and upper extremity arterial stenosis that reduces arterial flow. PAD is well known to be associated with increased morbidity and mortality in patients with cardiovascular (CV) disease.
Many people with PAD have no symptoms. The most common peripheral artery disease symptoms of lower-extremity PAD is painful muscle cramping in the hips, thighs, or calves when walking, climbing stairs, or exercising. Those who experience a painful ache in their legs while walking, on the other hand, usually recover after a few minutes of rest. This is referred to as “intermittent claudication” in medicine.
Atherosclerosis is among the major peripheral artery disease causes. Atherosclerosis affects the arteries all over the body. It causes peripheral artery disease when it occurs in the arteries that supply blood to the limbs.
For peripheral artery disease diagnosis, physical examination of the legs is performed to look for symptoms such as shiny skin, brittle toenails, hair loss on the legs and feet, and leg ulcers. PAD is commonly diagnosed using the ankle-brachial pressure index (ABPI) test. When the diagnosis is uncertain, additional testing, such as an ultrasound scan or an angiogram, is performed.
XARELTO (rivaroxaban) (Bayer/Johnson & Johnson), ZONTIVITY (Vorapaxar) (Xspire Pharma/Aralez Pharmaceuticals/Merck), and COLLATEGENE (beperminogene perplasmid) (AnGes, Inc/Mitsubishi Tanabe Pharma) are the FDA approved peripheral artery disease medications available in the market.

Downloads
Article in PDF
Recent Articles
- First Patient Enrolled in Kaizen Clinical Study in Japan; Koneksa’s At-Home Mobile Spirometry Cli...
- Business CockTail
- Asahi Intecc Partners with Penumbra; Prosoma and EVERSANA Announce Commercialisation Partnership;...
- Mindray’s TE Air Handheld Ultrasound System; Getinge’s iCast™ Covered Stent System; Clinic...
- Alcon’s Dry Eye Treatment Device; I-VASC Secures Series A Funding; Paige’s AI Medical Device Soft...