KalVista’s EKTERLY: First-Ever Oral Hereditary Angioedema Treatment
Jul 14, 2025
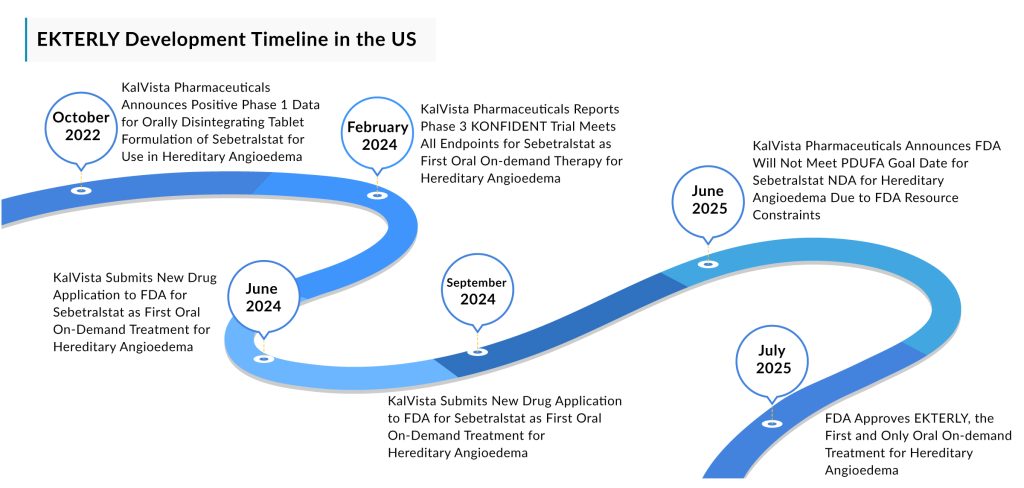
Following an unexpected regulatory delay last month, KalVista Pharmaceuticals has officially entered the commercial market with the FDA approval of its plasma kallikrein inhibitor, sebetralstat. On July 7, 2025, the FDA cleared sebetralstat, now branded as EKTERLY, as an oral treatment for acute hereditary angioedema (HAE) attacks in patients aged 12 and older. EKTERLY becomes the first and only oral, on-demand therapy available for HAE. This approval came shortly after the agency missed its initial target decision date of June 17.
Sebetralstat received Fast Track and Orphan Drug Designations from the US FDA, as well as Orphan Drug Designation and an approved Pediatric Investigational Plan from the EMA. KalVista continued to build on the extensive clinical evidence demonstrating the efficacy and safety of sebetralstat for HAE.
KalVista had previously attributed the delay to the FDA’s “heavy workload and limited resources,” referencing feedback from the agency. The holdup added to broader concerns about the FDA’s operational capacity, which has been under pressure following major staffing cuts at the Department of Health and Human Services (HHS) and its sub-agencies. Now moving past the delay, KalVista plans to roll out EKTERLY in the US market without further delay, marking the Massachusetts-based biotech’s first commercial product.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- The Expanding Market of Complement Inhibitors
- Novartis gets CAR-T drug; Shire aims to block; Xeljanz stands; Payer snubs; Lawmakers pass a bill
- Major Highlights and Insights from the AAAAI Annual Meeting, 2024
- Merck’s WINREVAIR Granted FDA Priority Review for Pulmonary Arterial Hypertension; KalVista’s EKT...
- Hereditary Angioedema (HAE) – Active drug pipeline
“The FDA’s approval of EKTERLY is a milestone for people living with HAE,” said KalVista CEO Ben Palleiko. “It allows patients to address symptoms as soon as they appear, wherever they are. This achievement reflects the strength of our science and our commitment to the HAE community. I’m deeply thankful to the KalVista team, patients, healthcare providers, and advocacy groups like HAEA and HAEi for their support. We believe EKTERLY could become a cornerstone treatment for HAE, and we are now focused on making it available to those who need it.”
HAE is a rare genetic disorder caused by a deficiency or dysfunction of the C1 esterase inhibitor (C1INH) protein. According to DelveInsight, there were approximately 14,000 diagnosed prevalent HAE cases across the 7MM in 2024, a number expected to grow significantly by 2034. Among the 7MM, the US accounted for the highest prevalent cases of HAE in 2024, while Japan accounted for the least number of prevalent cases. The type-specific diagnosed prevalent cases of HAE are categorized into Type I, Type II, and HAE with normal C1-INH (Type III). Among these, Type I HAE accounted for the highest cases in the 7MM in 2024.
Before EKTERLY’s approval, all FDA-approved on-demand HAE treatments in the US required intravenous or subcutaneous administration, creating a notable treatment burden. Despite the availability of prophylactic therapies, many patients still experience unpredictable attacks and need fast access to effective on-demand treatment.
Despite the availability of several approved treatments for hereditary angioedema in the US, including Takeda’s TAKHZYRO and CSL Behring’s HAEGARDA, BERINERT 2000/3000, and ANDEMBRY, for both acute attacks and prevention, KalVista’s oral, on-demand therapy offers a simpler dosing approach that could position EKTERLY as a cornerstone treatment for the condition.
The FDA approved EKTERLY based on findings from KalVista’s phase III KONFIDENT trial, the largest clinical study ever conducted in HAE. Published in the New England Journal of Medicine in May 2024, the data demonstrated that EKTERLY provided significantly faster symptom relief, reduced attack severity, and improved attack resolution compared to placebo, while maintaining a safety profile comparable to placebo. The trial enrolled 136 patients across 66 sites in 20 countries.
Additional support came from the ongoing KONFIDENT-S open-label extension trial, which, as of September 2024, showed that patients were able to treat HAE attacks within a median of 10 minutes of symptom onset. Furthermore, the latest KONFIDENT-S results indicated a median time to symptom relief of 1.3 hours in attacks involving the larynx, abdomen, or breakthrough episodes in patients on long-term prophylaxis. The safety data from more than 1,700 treated attacks in KONFIDENT-S confirmed a safety profile consistent with that observed in the KONFIDENT trial for the 600 mg dose of EKTERLY.
KalVista is set to launch EKTERLY in the US right away, allowing physicians to start prescribing the treatment today. To support patients, the company has introduced KalVista Cares, a comprehensive patient assistance program providing tailored services and resources for eligible individuals. This program offers help with understanding insurance coverage, support for accessing the medication, and continuous assistance throughout the course of treatment.
Although EKTERLY’s approval has allowed KalVista to enter the HAE treatment landscape, but it will face fierce competition from the other companies working on their lead asset. One such company is Ionis Pharmaceuticals, which is working on its candidate Donidalorsen.
Donidalorsen, previously referred to as IONIS-PKK-LRx, is an experimental RNA-based therapy aimed at reducing the production of prekallikrein (PKK), a key factor involved in triggering inflammatory pathways that cause acute hereditary angioedema attacks. By lowering PKK levels, donidalorsen has the potential to serve as a preventive treatment for HAE attacks, pending regulatory approval. The drug is currently undergoing regulatory review in the United States, with a Prescription Drug User Fee Act (PDUFA) decision expected by August 2025.
Another company working in the space is Intellia Therapeutics. Intellia Therapeutics is developing NTLA-2002, an experimental CRISPR-based therapy aimed at disabling the kallikrein B1 (KLKB1) gene, which produces prekallikrein, a precursor of the kallikrein protein. The therapy has received Orphan Drug Designation (ODD) from the European Union. Intellia shared data from the Phase II segment of the trial in the second half of 2024, with results also published in the New England Journal of Medicine (NEJM). In January 2025, the company announced that the first patient was dosed in its global Phase III trial for hereditary angioedema. Intellia anticipates completing patient enrollment in the second half of 2025 and plans to submit a Biologics License Application (BLA) in 2026, targeting a US launch in 2027.
Meanwhile, Navenibart, Astria Therapeutics’ lead product, is an experimental monoclonal antibody designed to inhibit plasma kallikrein. In February 2025, Astria Therapeutics launched the ALPHA-ORBIT Phase III pivotal trial of Navenibart for the treatment of hereditary angioedema. As outlined in the company’s March 2025 corporate presentation, Astria plans to share data from the ALPHA-SOLAR study in mid-2025.
Overall, potential HAE therapies are being investigated for the treatment of hereditary angioedema, and it is safe to predict that the hereditary angioedema treatment space will significantly impact the market in the coming years.
It will also be interesting to see how EKTERLY maintains its dominance in the oral HAE treatment space and how the anticipated launch of the emerging HAE therapies will change the dynamics of the therapeutic domain.

Downloads
Article in PDF
Recent Articles
- Novartis gets CAR-T drug; Shire aims to block; Xeljanz stands; Payer snubs; Lawmakers pass a bill
- BridgeBio bags $299M; Immunochina receives $20M; Attune raises; Wren receives $23M
- Merck’s WINREVAIR Granted FDA Priority Review for Pulmonary Arterial Hypertension; KalVista’s EKT...
- Celltrion Announces FDA Nod for New STEQEYMA Presentation to Broaden Pediatric Use; CSL’s ANDEMBR...
- Hereditary Angioedema (HAE) – Active drug pipeline