Analyzing Strategies in Polymyositis and Dermatomyositis Treatment
Jul 17, 2023
Inflammatory myositis refers to a group of disorders affecting the muscles and may be associated with different causes such as infection, drug toxicity, trauma, and autoimmune diseases. The most prominent cause of myositis is an autoimmune disease, typically known as idiopathic inflammatory myopathy (IIM). The indication is subdivided into Polymyositis, Dermatomyositis, Inclusion body myositis (IBM), Immune-Mediated Necrotizing Myopathy (IMNM), Anti-synthetase syndrome (ASyS), and Overlapping myositis (OS).
Polymyositis, Dermatomyositis, and Inclusion body myositis were the only subcategories known for a long time. But with recent advancements and the broadening of diagnostic criteria and approaches, additional classes have been added to the list. Polymyositis and Dermatomyositis still form a significant patient pool and are the major focus for most pharmaceutical companies in the domain.
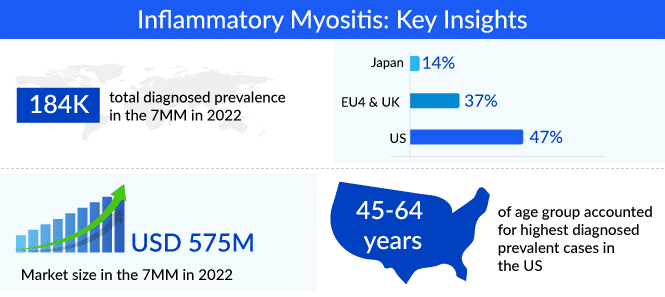
By definition, polymyositis is a rare inflammatory condition that affects the muscles of both sides of the body making it difficult for the patient to perform basic voluntary muscle movements, whereas dermatomyositis is marked by muscle weakness in addition to skin rashes, with the heliotrope rash a characteristic symptom. The two diseases affect more than 60,000 people in the US and have a female preponderance. A detailed epidemiological analysis of dermatomyositis and polymyositis for the 7MM countries is available in Delveinsight’s Inflammatory Myositis Market Forecast report.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Leading Autoimmune Diseases to Undergo Robust Improvement in Treatment by Major Pharma Companies
- Merck to Acquire Harpoon Therapeutics; Novo Nordisk Enters Into Collaborations with Omega Therape...
- ANCA-associated Vasculitis Treatment Market: Unraveling the Complexities
- Cytokinetics Announces Results From SEQUOIA-HCM Clinical Trial of Aficamten; FDA Approves Chiesi’...
- Which Key Player Holds the Potential to Corner the Rheumatoid Arthritis Therapeutics Market?
Current Inflammatory Myositis Treatment Market Outlook
Polymyositis and Dermatomyositis do not currently have cures. As they are mainly associated with autoimmune diseases, the first line of therapy involves the use of corticosteroids, either given as monotherapy or along with immune suppressants. A second line of therapy for patients that relapse includes the use of immune suppressants, mainly methotrexate, azathioprine, cyclophosphamide, etc. Immunoglobulin therapy may also be used where intravenous (IVIG) antibodies are administered. Octagam 10% is one such IV immunoglobulin approved for adult dermatomyositis. In the case of extremely severe or refractory cases, biologics such as Rituximab are also considered.
While clinical practice has evolved to manage the disease over the years, there is still no drug that is FDA-approved as a specific cure for idiopathic inflammatory myopathy. Most treatments are off-label and there is a lack of inflammatory myositis treatment guidelines, with doctors having to rely on intuitive approaches sometimes with limited success. A constant need to change lines of treatment and an incremental number of relapsed patients results in a burgeoning burden on doctors and the healthcare system. In addition, the long-term prognosis of success of the therapies is poor due to side effects and insufficient control of symptoms and the morbidity/mortality rate associated with the disease is high. With all these factors, there poses a significant unmet need for approved therapies in the inflammatory myositis treatment market.
Key Player’s Considerations and their Emerging Therapy Prospects in the Inflammatory Myositis Treatment Market
The unmet inflammatory myositis treatment need is just one factor that will drive launch performance for the drugs under development. Pharmaceutical companies must bring out differentiating benefits in terms of superior safety and efficacy results, speed to inflammatory myositis market, and broader target patient pools wherever possible to maximize market opportunity. The complex nature of the idiopathic inflammatory myopathy disease makes it imperative to target myositis patients across sub-types as symptoms overlap and differential diagnosis is challenging.
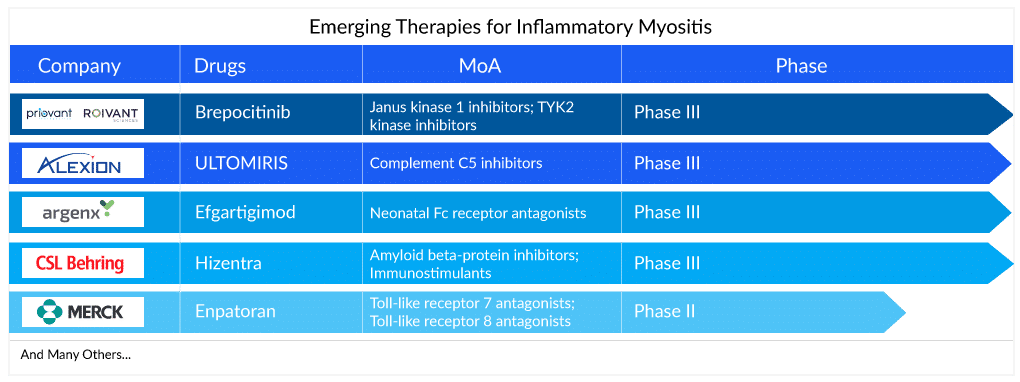
A recent molecule being developed for the therapy area is Brepocitinib, a Phase III drug from Pfizer and Priovant Therapeutics, which is a dual selective inhibitor of TYK2 and JAK1 inhibitor. Being a JAK inhibitor, it would exert immune-suppressive effects and is being evaluated as a potential treatment for dermatomyositis as well as other severe autoimmune diseases. The drug is particularly promising owing to its targeted MoA and oral route of administration and is likely to hit the market by 2026 in the US and reach a peak revenue of around USD 350 million.
Additionally, Argenx is developing a drug, Efgartigimod alfa-fcab (ARGX-113), currently in Phase II/III, for inflammatory myositis treatment including Dermatomyositis, Polymyositis (including ASyS) and immune-mediated necrotizing myopathy (IMNM). The drug is likely to be launched in 2028 and is expected to attain peak sales of around USD 500 million in the 7MM countries, as per analysts at Delveinsight. Although a relatively late-entrant into the market, its novel Fc receptor-blocking mechanism of action and the fact that it targets a broader IIM patient pool will bolster its success in the inflammatory myositis treatment market. Moreover, the monoclonal antibody is already approved for myasthenia gravis with good efficacy and long-lasting effect in comparison to standard of care.
There is also a focus on the high proportion of relapsed cases where aggressive symptom management is common. Ravulizumab, a drug by Alexion Pharmaceuticals, has already been approved for paroxysmal nocturnal hemoglobinuria (PNH) treatment and is under development for dermatomyositis. Owing to the fact that it is a biologic and its cost is high, the drug is being considered for relapsed patients of dermatomyositis and is estimated to reach a peak sales of around USD 500 million in the 7MM, as per the Inflammatory Myositis Market report by Delveinsight.
Analyst assessment
While pharmaceutical companies focus on the imminent launches of their drugs in a relatively untapped inflammatory myositis treatment market, it is necessary to continuously reassess their decisions on when and how to bring these inflammatory myositis drugs into the market. Finding the right balance between the values of the drug attributes and addressing the unmet medical need in the market will determine the drugs’ success at launch and shape the inflammatory myositis treatment market going forward.

FAQ
Inflammatory myositis, also known as myositis, is a group of rare autoimmune disorders characterized by inflammation of the muscles. There are several types of inflammatory myositis, including polymyositis, dermatomyositis, inclusion body myositis, and necrotizing autoimmune myopathy.
The exact cause of inflammatory myositis is unknown, although it is thought to be related to an aberrant immunological response in which the body’s immune system destroys its own muscle tissue by mistake.
Muscle weakness, muscle soreness, difficulty swallowing, weariness, and, in certain cases, skin rashes are the most common symptoms of inflammatory myositis. The severity of the inflammatory myositis symptoms varies and may worsen over time.
A medical history, physical examination, blood tests, electromyography (EMG), muscle biopsy, and imaging techniques such as MRI are commonly used to diagnose inflammatory myositis.
The goal of inflammatory myositis treatment is to inhibit the immune system and minimize inflammation in order to relieve symptoms and avoid additional muscle damage. To maintain muscle strength and function, corticosteroids, immunosuppressive medications, and physical therapy are frequently used in inflammatory myositis treatment.
Downloads
Article in PDF
Recent Articles
- Harnessing the Power of Immunomodulators: Applications and Implications
- ANCA-associated Vasculitis Treatment Market: Unraveling the Complexities
- Unraveling the Complexities of Dermatomyositis Treatment: A Comprehensive Review and Future Persp...
- Sanofi’s Rare Disease Drug Xenpozyme’ Approval; FDA Approves Novartis’ Pluvicto; AstraZenec...
- Which Key Player Holds the Potential to Corner the Rheumatoid Arthritis Therapeutics Market?