Evolving Therapies in Kidney Transplant Rejection Treatment: From Rejection to Recovery
Jul 25, 2025
Kidney transplant rejection remains one of the most significant challenges in the field of organ transplantation, despite remarkable advances in surgical techniques and immunosuppressive therapies. Around 10–15% of kidney transplant recipients experience acute rejection episodes within the first year, which, if left untreated, can lead to graft failure and a return to dialysis.
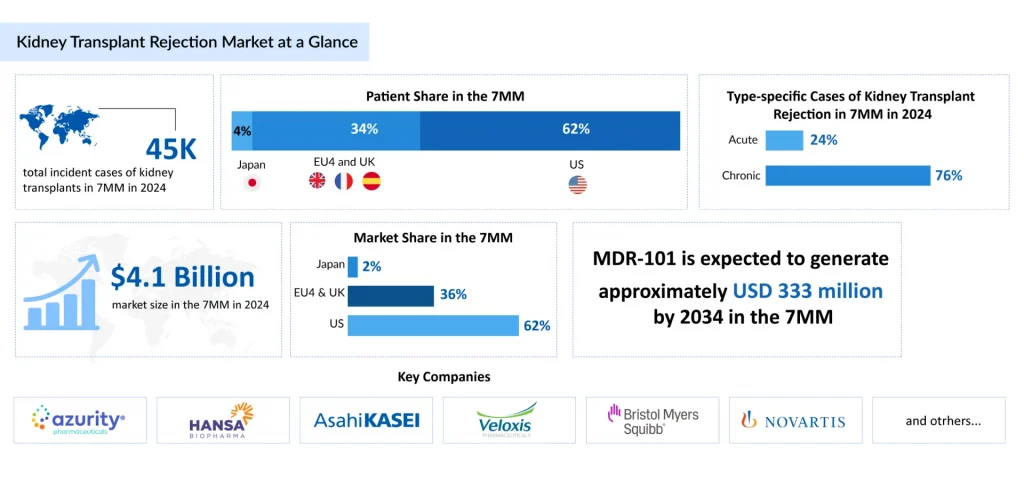
In 2024, there were an estimated 45K total incident cases of kidney transplants across the 7MM, and it will reach up to 70K by 2034 as per DelvsInsight analysis. Among the 7MM, the US accounted for approximately 55% of the living cases of kidney transplants in 2023.
This silent battle between the body and the transplanted organ underscores the importance of early detection, precise monitoring, and innovative treatments.
Downloads
Click Here To Get the Article in PDF
From ENVARSUS XR to SIMULECT: Medications Shaping Transplant Outcomes
Kidney transplant rejection remains a major challenge in post-transplant care, necessitating the use of effective immunosuppressive therapies to prevent rejection and ensure long-term graft function. A range of approved kidney transplant rejection medications is available to manage kidney transplant rejection, each targeting specific pathways of the immune system. Among them, MYHIBBIN (mycophenolate mofetil oral suspension), approved by the US FDA in 2024, is an oral small-molecule drug that inhibits inosine monophosphate dehydrogenase (IMPDH), a key enzyme for purine synthesis and lymphocyte proliferation. It is primarily indicated for preventing acute rejection in kidney transplant recipients.
IDEFIRIX (imlifidase), a biologic approved in the EU in 2020 and designated as an Orphan Drug in the US, works by cleaving immunoglobulin G (IgG), thereby suppressing the immune response that leads to transplant rejection. Administered intravenously, IDEFIRIX is particularly beneficial for patients with elevated donor-specific antibodies (DSAs). THYMOGLOBULIN (anti-thymocyte globulin [rabbit]), developed by Sanofi, is a polyclonal antibody that depletes T cells to lower the risk of acute rejection. Approved in the US in 2017 and earlier in the EU and Japan, THYMOGLOBULIN is widely used during the induction phase of transplantation and also carries US Orphan Drug Designation.
Another key kidney transplant rejection therapy is ENVARSUS XR (tacrolimus extended-release), a calcineurin inhibitor by Asahi Kasei, approved in the US (2015) and EU (2014). The FDA expanded its approval in 2018 for de novo kidney transplant patients. By inhibiting T cell activation, ENVARSUS XR prevents organ rejection and provides an extended-release formulation that improves patient adherence compared to standard tacrolimus. NULOJIX (belatacept), from Bristol Myers Squibb, is a biologic agent that blocks T cell activation by binding to the CD80/86 receptor on antigen-presenting cells. Approved in 2011, it offers an alternative to calcineurin inhibitors, particularly for patients at risk of nephrotoxicity, and holds Orphan Drug Designation in the US.
Lastly, SIMULECT (basiliximab), a monoclonal antibody approved globally in the late 1990s, prevents acute rejection by blocking interleukin-2 receptors critical for T cell activation. It is often administered alongside other immunosuppressive therapies to minimize early organ rejection. Collectively, these drugs, with their unique mechanisms of action and delivery methods, form the cornerstone of kidney transplant rejection management.
Key Contenders in the Kidney Transplant Rejection Pipeline
The kidney transplant rejection pipeline features several promising drug candidates, including MDR-101 from Medeor Therapeutics, Tegoprubart (AT-1501) from Eledon Pharmaceuticals, and Riliprubart (BIVV020, SAR445088) from Sanofi, among others.
MDR-101, developed by Medeor Therapeutics, is a cellular therapy derived from a living kidney donor’s blood and peripheral stem cells. Its primary goal is to induce donor-specific immune tolerance, thereby preventing kidney transplant rejection. By potentially eliminating the need for lifelong immunosuppressive drugs and their associated side effects, MDR-101 aims to improve both kidney function and transplant survival rates. The therapy works by reprogramming the recipient’s immune system to accept the donor organ, offering a transformative approach to long-term kidney transplantation outcomes. In November 2023, Medeor Therapeutics announced encouraging interim results from a Phase III trial of MDR-101, which were highlighted during a late-breaking oral presentation at the American Society of Nephrology (ASN) Kidney Week 2023 Annual Meeting.
Tegoprubart (AT-1501), a product of Eledon Pharmaceuticals, is a humanized monoclonal antibody designed to target CD40L. By blocking the CD40-CD40L interaction, it introduces a novel immunosuppressive strategy aimed at replacing traditional Calcineurin Inhibitors (CNIs) such as tacrolimus. Eledon completed patient enrollment for the Phase II BESTOW trial of tegoprubart in kidney transplantation in September 2024, four months ahead of schedule, with topline results anticipated in the fourth quarter of 2025.
Riliprubart (BIVV020, SAR445088), developed by Sanofi, is an IgG4 humanized monoclonal antibody under investigation for the treatment of antibody-mediated rejection. It works by selectively inhibiting activated complement component C1s, a key player in the inflammatory pathways driving AMR. The drug is currently being evaluated in Phase II clinical trials for patients who are either at risk of or diagnosed with AMR.
The anticipated launch of these emerging therapies are poised to transform the kidney transplant rejection treatment landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the kidney transplant rejection treatment landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
Kidney Transplant Rejection Prophylaxis Market on an Upward Trajectory
The kidney transplant rejection treatment landscape is undergoing significant advancements, with current strategies focusing on immunosuppressive therapies, monitoring techniques, and supportive care aimed at preventing rejection and maintaining graft function. In 2024, the kidney transplant rejection prophylaxis market across the 7MM was valued at USD 4.1 billion and is projected to grow over the forecast period (2025–2034).
Advances in immunosuppressive therapies and improved diagnostic monitoring have significantly enhanced graft survival rates, boosting the demand for effective prophylactic treatments. Moreover, the growing adoption of novel biologics and targeted agents, alongside better patient stratification strategies, is expanding the market potential. With a growing awareness among healthcare providers and patients about the importance of early and sustained prophylaxis, the kidney transplant rejection market is expected to witness steady growth over the forecast period.
Market expansion is further supported by the development of next-generation immunomodulators that offer improved safety and efficacy profiles compared to conventional therapies such as calcineurin inhibitors and corticosteroids. Pharmaceutical companies are investing in innovative pipeline products aimed at minimizing adverse effects and reducing the risk of chronic rejection, which remains a major challenge post-transplantation. Favorable reimbursement frameworks, coupled with the rising healthcare expenditure in both developed and emerging economies, are also expected to accelerate kidney transplant rejection treatment market growth.
Additionally, the kidney transplant rejection treatment market is being shaped by collaborations between biopharmaceutical companies, research institutions, and transplant centers to develop precision medicine approaches and combination therapies for transplant patients. The integration of advanced monitoring techniques, such as biomarker-based assays and non-invasive molecular diagnostics, is further enhancing patient outcomes and supporting the long-term success of kidney transplantation. These factors, combined with an increasing global transplant population, are likely to sustain the kidney transplant rejection market’s upward momentum in the coming years.

Downloads
Article in PDF