Next-Gen Spinal Cord Injury Therapies: A Look at the Evolving Drug Pipeline
Jul 18, 2025
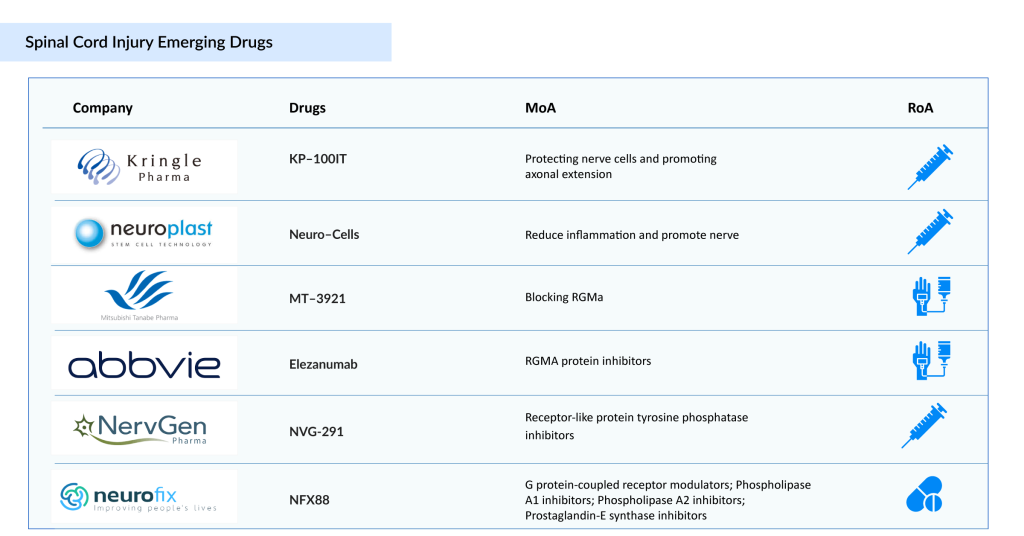
The treatment options for spinal cord injury remain limited, with few approved spinal cord injury therapies available. At present, STEMIRAC is the only approved SCI treatment, and it is offered exclusively in Japan. There is an urgent need for targeted solutions that can enhance patient outcomes and support functional recovery. The SCI therapeutic pipeline is steadily growing, with promising candidates such as Kringle Pharma’s KP-100IT, Neuroplast’s Neuro-Cells, Mitsubishi Tanabe Pharma America’s MT-3921, AbbVie’s Elezanumab, Pharmazz’s PMZ-1620, Neurofix’s NFX88, NervGe n Pharma’s NVG-291, ReNetX Bio’s AXER-204, and NurExone’s ExoPTEN, among others. The introduction of these innovative spinal cord injury therapies is expected to revolutionize the SCI treatment landscape in the coming years. Let’s explore the 6 key spinal cord injury therapies that hold the potential to redefine the future of spinal cord injury care.
Kringle Pharma’s KP-100IT
KP-100IT, developed by Kringle Pharma, is an intrathecal formulation of Hepatocyte Growth Factor (HGF) aimed at treating acute spinal cord injury. The spinal cord injury therapy works by protecting nerve cells and promoting axonal regeneration, offering potential to enhance motor function recovery in affected patients. Research in acute SCI has accelerated, driven by both regulatory and academic initiatives.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- The Spinal Cord Injury Treatment Landscape: Limited Progress, Immense Potential
- Late-Breaking Science at AAN 2025: Shaping the Future of Neurology
- Navigating the Emerging Trends and Technologies in the Spinal Cord Injury Rehabilitation Segment
- MEDIT’s i700 Intraoral Scanner; Phantom Neuro and Blackrock’s Next-Generation Assistive Devices; ...
- Gene and Cell Therapies in CNS Disorders: Miracle Cure? Opportunities Galore!
The Japanese Ministry of Health, Labour and Welfare (MHLW) granted orphan drug designation (ODD) in 2019, and Keio University initiated a joint research project in 2024 to identify biomarkers for predicting spontaneous recovery. Additionally, Kringle Pharma signed an agreement in 2021 with Keio University School of Medicine to advance HGF-based therapies.
In March 2024, Keio University published a study on gene expression profiles following HGF administration in acute SCI, providing insights into its therapeutic potential. In February 2024, Kringle Pharma reported topline results from a Phase III trial of KP-100IT in acute SCI. Furthermore, in June 2025, the FDA granted orphan drug designation to KP-100IT.
Neuroplast’s Neuro-Cells
Neuro-Cells is an advanced stem cell therapy in development for Traumatic Spinal Cord Injury (TSCI). Sourced from the patient’s own bone marrow, the treatment aims to reduce inflammation and stimulate regeneration in the central nervous system, ultimately working to preserve and restore function, mobility, and independence.
In May 2019, the European Medicines Agency (EMA) granted Orphan Drug Designation (ODD) for its use in traumatic SCI. By September 2023, Neuroplast had completed patient enrollment for its Phase II clinical trial assessing Neuro-Cells in TSCI, which demonstrated an excellent safety profile with no treatment-related adverse events and positive patient feedback indicating strong tolerability.
Currently, the therapy is being investigated in Phase II/III clinical trials for TSCI under the authorization of the Spanish Medical Ethical Committee (CEIm) and the national regulatory body, Agencia Española de Medicamentos y Productos Sanitarios (AEMPS).
Mitsubishi Tanabe Pharma America’s MT-3921
MT-3921 (unasnemab) is a humanized monoclonal antibody developed by Mitsubishi Tanabe Pharma Corporation (MTPC) in collaboration with Osaka University. It targets repulsive guidance molecule A (RGMa), a key inhibitor of neuronal survival and regeneration, positioning it as a potential therapy for spinal cord injury (SCI).
In July 2021, the US FDA granted Fast Track Designation (FTD) to MT-3921, enabling accelerated communication and review to support its development for SCI. Following this, a Phase II Proof-of-Concept trial was launched in September 2021, enrolling SCI patients at clinical sites in the US, Japan, and other regions.
AbbVie’s Elezanumab
Elezanumab is a human IgG1 monoclonal antibody that specifically targets repulsive guidance molecule A (RGMa). RGMa acts as an inhibitor of axonal growth and is a key factor in preventing neuronal regeneration and functional recovery after central nervous system (CNS) injury. Elezanumab is being developed as a potential therapy for spinal cord injuries, multiple sclerosis, and acute ischemic stroke.
It is currently undergoing evaluation in a phase II clinical trial (NCT04295538) for spinal cord injury treatment. In September 2020, AbbVie announced that the FDA granted Orphan Drug and Fast Track designations to elezanumab (ABT-555) as an investigational therapy for patients with spinal cord injury.
Neurofix’s NFX88
NFX88 is an innovative drug developed to alleviate neuropathic pain commonly experienced by patients with spinal cord injuries. Featuring a distinctive lipid-based structure (2-hydroxyoleic acid), NFX88 is uniquely designed to target and relieve neuropathic pain by interacting directly with cell membranes. Unlike traditional treatments, its mechanism of action modifies the molecular composition and physicochemical properties of these membranes.
This interaction triggers a series of intracellular changes, reducing the production of proinflammatory substances (such as lysophosphatidic acid and phospholipase A) while promoting the expression of molecules involved in neuronal growth and repair (including GDF10 and TNC).
Clinical trials have already shown positive outcomes: Phase I (NCT01792310) confirmed the drug’s tolerability, while Phase IIA (2018-004792-13) demonstrated a strong safety profile and significant therapeutic efficacy. Notably, many patients reported substantial pain relief without associated adverse effects.
With Phase IIA successfully completed, Neurofix Pharma is now preparing for the next stage, Phase IIB/III, expected to begin later this year. This pivotal trial will involve approximately 30 hospitals across multiple European Union countries and represents the final step toward commercialization, pending favorable results.
NervGen Pharma’s NVG-291
NervGen holds exclusive global rights to NVG-291, a first-in-class therapeutic peptide designed to promote nervous system repair. The technology behind NVG-291 is licensed from Case Western Reserve University and builds on academic research demonstrating the preclinical efficacy of NVG-291-R, its rodent prototype, in animal models of spinal cord injury.
These studies suggest multiple molecular and cellular mechanisms through which NVG-291-R supports neurorepair and functional recovery in both central and peripheral nervous system injuries. Key mechanisms include enhancing neuronal sprouting (plasticity), promoting remyelination, and inducing a non-inflammatory phenotype in microglial cells. The FDA has granted NVG-291 Fast Track designation for spinal cord injury.
In June 2025, NervGen Pharma Corp. reported positive topline data from the chronic cohort (1–10 years post-injury) of its Phase 1b/2a clinical trial assessing NVG-291 as a potential therapy for spinal cord injury. Results reinforced NVG-291’s ability to facilitate nervous system repair, with the study meeting a co-primary endpoint demonstrating improved motor connectivity in participants with chronic cervical SCI treated with NVG-291 (n=10) compared to placebo (n=10).
Patients receiving NVG-291 showed a three-fold increase in motor connectivity strength to the first dorsal interosseus muscle, as measured by changes in normalized motor evoked potential (MEP) amplitude (Baseline/Week 12: 6.207/18.773 for NVG-291 vs. 6.527/7.760 for placebo; p=0.0155). The second co-primary endpoint, assessing leg muscle (tibialis anterior) connectivity, did not reach statistical significance. The trial design allowed for success if at least one co-primary endpoint achieved significance, with a more stringent p-value threshold of <0.025 applied.
In conclusion, numerous promising therapies are under investigation for SCI, and it is reasonable to expect that SCI treatment space will substantially influence the market in the coming years. As these advanced spinal cord injury therapies progress and secure regulatory approvals, they are anticipated to transform the SCI treatment landscape by setting new standards of care and driving both medical innovation and economic development.

Downloads
Article in PDF
Recent Articles
- The Spinal Cord Injury Treatment Landscape: Limited Progress, Immense Potential
- MEDIT’s i700 Intraoral Scanner; Phantom Neuro and Blackrock’s Next-Generation Assistive Devices; ...
- Gene and Cell Therapies in CNS Disorders: Miracle Cure? Opportunities Galore!
- Late-Breaking Science at AAN 2025: Shaping the Future of Neurology
- Navigating the Emerging Trends and Technologies in the Spinal Cord Injury Rehabilitation Segment