Limbal Stem Cell Deficiency (LSCD): A Multifactorial Disease with Challenging Diagnosis and Treatment
Dec 26, 2022
Limbal Stem Cell Deficiency (LSCD) is a rare, progressive ocular surface disorder that results in conjunctivalization and neovascularization of the corneal surface. Ocular discomfort, reduced visual acuity, and photophobia are all symptoms of LSCD, which can be brought on by severe ocular surface illness.
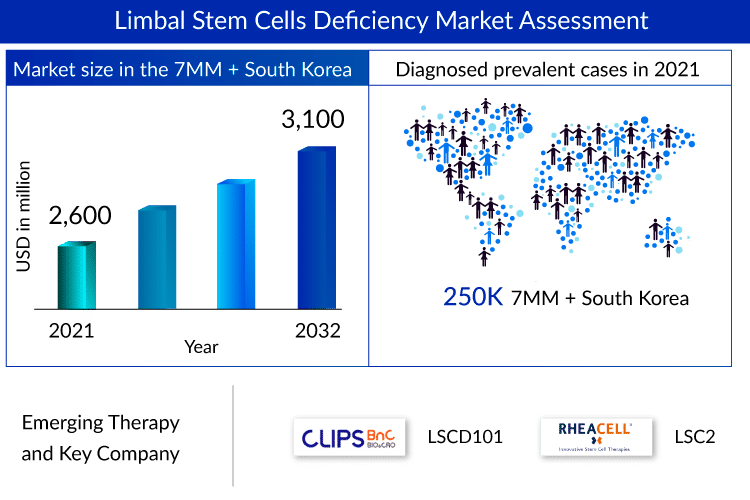
As per the assessment done by DelveInsight, the diagnosed prevalent cases of Limbal Stem Cell Deficiency (LSCD) associated with the 7MM + South Korea exceeded 250K in 2021.
The procedure that has been most frequently used to diagnose LSCD is slit-lamp biomicroscopy. A trusted technique for identifying disorders of the ocular surface is impression cytology. Goblet cells, a defining feature of LSCD, are seen on the cornea, indicating conjunctival cell invasion. In the current LSCD diagnosis system, the detection of goblet cells by impression cytology on samples collected from corneal surfaces has been regarded as the “gold standard.” Recent developments in diagnosing LSCD diagnosis include genetic techniques, anterior segment OCT, and in vivo imaging.
Downloads
Click Here To Get the Article in PDF
There is an unmet need for formal LSCD treatment guidelines, and treatment choice depends heavily on the severity of the condition. LSCD can be reversed if diagnosed and treated in its early stages. If misdiagnosed or not addressed, it can lead to total limbal deficiency requiring surgical intervention.
LSCD treatment has involved various approaches, all of which have a common objective; the regeneration of a self-renewing, transparent, and homogeneous epithelium on the corneal surface. These methods have frequently been developed through cooperation between ophthalmologists and stem cell translational scientists. An autologous or allogenic limbal stem cell transplant is done to repair the ocular surface in advanced stem cell deficient corneas.
Only HOLOCLAR (autologous human corneal epithelial cells incorporating stem cells) and OCURAL are approved to treat LSCD patients (human [autologous] oral mucosa-derived epithelial cell sheet). Concerning adult patients with moderate-to-severe LSCD brought on by burns, particularly chemical burns to the eyes, HOLOCLAR is commercially available in the EU nations. A “tissue-engineered product,” or advanced therapeutic product, known as HOLOCLAR, is made of cells extracted from the patient’s limbus (at the border of the cornea) and produced in a lab to restore the damaged corneal surface.
Ocural is a cutting-edge novel therapeutic option for patients with severe LSCD corneal damage in both eyes and significantly diminished visual acuity. Japan Tissue Engineering Co., Ltd. (J-TEC) declared in June 2021 that OCURAL had been given commercial approval in Japan for treating LSCD.
Numerous new resources are in the early stages of clinical testing for treating LSCD patients throughout many nations to meet the need. In our study period (2019–2032), early-stage products like LSCD101 (CliPS Co., Ltd.) and LSC2 (RHEACELL GmbH & Co. KG), among others, are anticipated to be released on the LSCD treatment market. These LSCD drugs are not approved globally, yet somehow, that does not seem to hinder a billion-dollar LSCD treatment market as they are highly-priced.
The high cost of stem cell therapies and transplantation and limited information about patients’ clinical and demographic features are two major factors that are likely to impede the LSCD treatment market success of the upcoming therapies.
LSCD market landscape with early-stage assets
The current perspective of the LSCD emerging pipeline landscape is scarce with early-stage assets, and the question remains about the successful approval by different regulatory authorities. The emerging LSCD therapies, although limited, have substantial potential. Among them, CliPS Co., Ltd.’s LSCD101 is in the pole position. Compared to the autologous limbal stem cell therapy (HOLOCLAR) commercialized in Europe, the limbal stem cell ability is superior to an average of about three times or more, and the safety has been proven as animal-derived cells and culture medium are excluded during cell culture.
Currently, a Phase II clinical trial of an autologous limbal stem cell therapy is in progress, and a clinical trial of an allogeneic limbal stem cell therapy is planned for patients with LSCD in both eyes. This also explains the interest of another company, RHEACELL GmbH & Co. KG, in these products to mark its presence in this high-priced market. Gearing up for this race, the next LSCD therapy on the list is LSC2. The membrane-bound ATP-binding cassette transporter, subfamily B, member 5 (ABCB5), originally described as a marker for dermal progenitor cells, was expressed on label-retaining (slow-cycling), p63α+ CK12− cells located in the basal limbal epithelium of mice and humans, respectively. This discovery identified ABCB5 as the first molecular surface marker for prospective LSC enrichment by antibody-based cell sorting.
As per DelveInsight’s analysis, the LSCD market is expected to reach ~USD 3,100 million by 2032.
Despite the promising LSCD pipeline, the aforementioned challenges cannot be ignored; however, at the molecular, cellular, and tissue levels, progress is being made to modify disease processes and lessen or completely eradicate blindness. For cell-based therapies to become an integral element of the therapeutic toolbox to combat corneal blindness, efforts must be coordinated from the most fundamental research to the most clinically focused studies. Because cell-based therapy for eye illnesses is one of the most effective instances of global regenerative medicine, we are unquestionably moving in the right direction.

FAQs
Limbal Stem Cell Deficiency (LSCD) is a rare, progressive ocular surface disorder that results in conjunctivalization and neovascularization of the corneal surface. Ocular discomfort, reduced visual acuity, and photophobia are all symptoms of LSCD, which can be brought on by severe ocular surface illness.
The procedure that has been most frequently used to diagnose LSCD is slit-lamp biomicroscopy. A trusted technique for identifying disorders of the ocular surface is impression cytology.
LSCD treatment has involved various approaches, all of which have a common objective; the regeneration of a self-renewing, transparent, and homogeneous epithelium on the corneal surface. Only HOLOCLAR (autologous human corneal epithelial cells incorporating stem cells) and OCURAL are approved to treat LSCD patients (human [autologous] oral mucosa-derived epithelial cell sheet).
The emerging LSCD therapies, although limited, have substantial potential. In our study period (2019–2032), early-stage products like LSCD101 (CliPS Co., Ltd.) and LSC2 (RHEACELL GmbH & Co. KG), among others, are anticipated to be released on the LSCD treatment market.
Downloads
Article in PDF