Evolving Landscape of Multiple System Atrophy Treatment: Recent Developments and Future Directions
Aug 22, 2025
Table of Contents
Multiple system atrophy (MSA) is a progressive neurodegenerative disorder characterized by a constellation of symptoms impacting both the autonomic nervous system and motor function. According to the National Organization for Rare Disorders (NORD), MSA affects between 15,000 and 50,000 individuals in the United States, affecting men and women across all racial groups. The etiology of MSA remains unknown, with the vast majority of cases occurring sporadically and without a clear cause. The peak onset typically occurs between the ages of 55 and 60, although onset can range from as early as 30 to beyond 90 years of age. NORD estimates the incidence of MSA in the US general population to be approximately 0.6 cases per 100,000 persons annually, corresponding to roughly 2,000 new diagnoses each year.
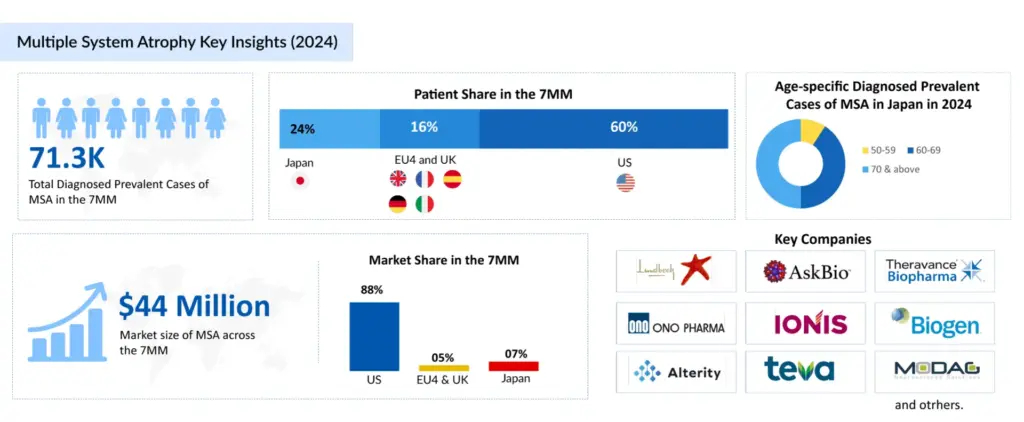
In the assessment done by DelveInsight, the estimated total diagnosed prevalent cases of multiple system atrophy in the 7MM were ~70K in 2024. The highest diagnosed prevalent cases of multiple system atrophy were found to be reported in the US compared to the other 7MM countries. As per DelveInsight’s estimates, the country alone accounts for ~60% of the total diagnosed prevalent cases among the 7MM countries. MSA includes two main subtypes: parkinsonian (MSA-P) and cerebellar (MSA-C), with MSA-P accounting for approximately 75% of all MSA cases, making it the more common form. Both subtypes cause significant disability, but MSA-P often resembles Parkinson’s disease, complicating diagnosis. Despite advances, there remains a substantial unmet need for effective treatments and early diagnostic tools for MSA. The rarity and complexity of the disease contribute to delayed diagnosis and limited therapeutic options, highlighting the urgency for enhanced research and targeted therapies to improve patient outcomes.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Teva’s AJOVY Expanded by FDA as First Anti-CGRP Preventive for Pediatric Episodic Migraine; NRx P...
- The Future of Parkinson’s Disease Treatment: Unlocking the Potential of Cell and Gene Therapy
- Dengvaxia study; Opdivo racks up; FDA issues plant; Teva’s Rimsa fraud; Mission bags Fox grant
- FDA Approves GSK’s Arexvy for RSV; CHMP’s Opinion on Gilead’s Hepcludex® for HDV; FDA Clearance t...
- Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
Neurodegenerative Connections: Similarities Between Multiple System Atrophy and Parkinson’s Disease
MSA and Parkinson’s disease are distinct neurodegenerative disorders but share clinical and pathological features, classifying them as synucleinopathies—marked by abnormal alpha-synuclein accumulation. Both present with motor symptoms like bradykinesia, rigidity, and postural instability, impacting daily functioning. However, MSA involves more widespread neurodegeneration, affecting the basal ganglia, cerebellum, and autonomic centers, leading to additional symptoms such as autonomic failure and ataxia. In contrast, Parkinson’s primarily targets dopaminergic neurons in the substantia nigra. While Lewy bodies are common to both, the broader distribution of neuronal damage in MSA accounts for its faster progression and more diverse symptom profile.
Breaking Ground: Promising MSA Treatment Approaches
Treatment for both MSA-P and MSA-C is primarily symptomatic. MSA-P may respond partially to Parkinson’s medications though benefits are often limited. MSA-C treatment lacks specific drugs, with care focused on managing balance and coordination issues through physical therapy, occupational therapy, and supportive interventions.
In the approved market segment for multiple system atrophy treatment, droxidopa (NORTHERA) is the world’s first approved medication for symptomatic nOH. Chelsea received approval for NORTHERA from the US FDA in February 2014 for symptomatic benefit in adult patients with nOH, which is found in the majority of multiple system atrophy patients and is a leading source of disability and injury in the disease. Since its approval, several generic versions of the drug are now available, increasing accessibility for patients requiring treatment for this condition.
There is currently no approved disease-modifying agent. The drug used to treat Parkinson’s disease, most notably levodopa (SINEMET), is also administered for multiple system atrophy patients. The efficacy of such drugs, however, varies substantially across affected individuals. Other drugs used to treat Parkinson’s disease, in addition to levodopa, may be used to treat multiple system atrophy patients. These included dopamine agonists like ropinirole (REQUIP) and pramipexole (MIRAPEXIN), which boost dopamine receptor activation in the brain. This aids the brain’s reception of dopamine messages. Midodrine hydrochloride (PROAMATINE) has been used to treat low blood pressure, which has been linked to multiple system atrophy in certain cases. Low blood pressure can be treated using adrenergic medications such as ephedrine. Low blood pressure can also be treated with L-threo-dihydroxyphenylserine (L-DOPS or Lthreo-DOPS). Midodrine is considered more physiologic and better tolerated than fludrocortisone, while other medicines worth exploring include indomethacin and intranasal desmopressin.
The breadth of treatment options for orthostatic hypotension in multiple system atrophy provides clinicians with some hope of alleviating the misery of many patients. Multiple system atrophy covers the major market with off-label and generic treatments. Clonazepam, vitamin E, propranolol, baclofen, or amantadine have demonstrated superior efficacy. However, in an open-label experiment with MSA-C patients, buspirone (off-label) improved upper-limb ataxia. Depending on the severity of the other symptoms, drugs such as sildenafil (VIAGRA), tadalafil (Cialis), or vardenafil (LEVITRA) may be used to treat urinary and erectile dysfunction (ED) symptoms that are common in males with multiple system atrophy.
Promising Therapies and Future Scenarios for Multiple System Atrophy Treatment
Considering all the existing knowledge gaps and the void of effective neuroprotective treatment, some major pharma players are proactive with their early and mid-phase trial candidates in the pursuit of a breakthrough therapy to dominate the multiple system atrophy treatment market. H Lundbeck A/S (Lu AF82422), Theravance Biopharma (Ampreloxetine), AstraZeneca/Takeda Pharma (TAK-341/MEDI1341), Ono Pharma (ONO-2808), Teva Pharmaceutical/MODAG GmbH (Emrusolmin), Ionis Pharmaceuticals, Inc./Biogen (ION464), Alterity Therapeutics (ATH434), and others are the leading companies working in the segment with their lead assets in different multiple system atrophy clinical trials.
The human Monoclonal Antibody (mAb) Amlenetug (Lu AF82422) targets the toxic alpha-synuclein protein, which is a pathogenic characteristic of multiple spinal atrophy. To eliminate these toxic proteins, the chemical operates similarly to the body’s natural immune system. Lu AF82422 may potentially reduce or stop the progression of MSA because it tackles the disease’s underlying biology, according to Lundbeck. In February 2025, Lundbeck announced that the US FDA has granted Fast Track designation to amlenetug. Previously, in April 2024, the US FDA granted Orphan Drug Designation (ODD) to Lundbeck’s Lu AF82422 for the treatment of MSA. Similarly, the European Medicines Agency (EMA) granted Lu AF82422 ODD status in April 2021.
Theravance Biopharma’s ampreloxetine is a once-daily Norepinephrine Reuptake Inhibitor (NRI) in development for the treatment of patients with symptomatic neurogenic orthostatic hypotension (nOH). It binds to norepinephrine transporters with a high affinity. Ampreloxetine increases extracellular norepinephrine concentrations by inhibiting the activity of these transporters. Theravance Biopharma recently presented updated Phase 3 data on ampreloxetine at the International MSA Congress in May 2025. The REDWOOD 0170 study showed that MSA patients experienced meaningful improvements in neurogenic orthostatic hypotension (nOH) symptoms after 16 weeks of ampreloxetine treatment. Symptoms remained stable in the treatment group but worsened with placebo. Functional activities like standing and walking improved and declined after placebo withdrawal. Despite existing therapies, MSA patients continue to have a high nOH symptom burden, highlighting an unmet need. These results reinforce ampreloxetine’s potential as a promising treatment for nOH in MSA.
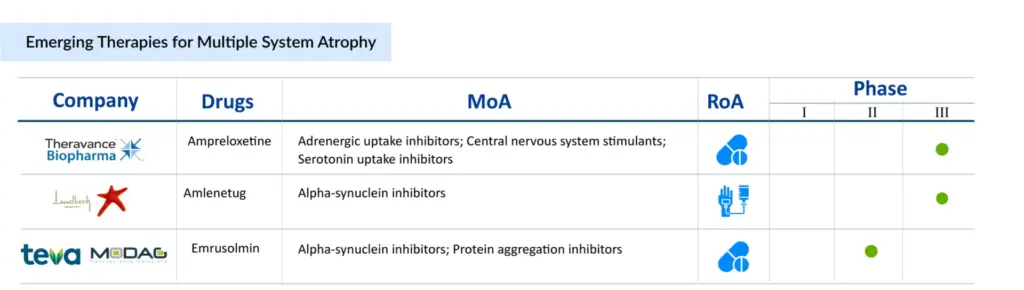
Alterity’s lead candidate, ATH434, is the first of a new generation of small molecules designed to block the aggregation of pathogenic proteins implicated in neurodegeneration. ATH434 decreases the aberrant accumulation of S and tau proteins in animal illness models by restoring normal iron balance in the brain. As a result, it has a high potential for treating various kinds of atypical Parkinsonism. Recently, Alterity Therapeutics announced that the first patient has been enrolled in new Phase II clinical trial investigating ATH434 in individuals with multiple system atrophy (MSA), a type of atypical parkinsonism. The open-label, biomarker ATH434-202 research (NCT05864365) at Vanderbilt University Medical Centre in Tennessee is enrolling 15 persons with advanced MSA and clinical indications of parkinsonism.
With the expected launch of these emerging therapies, the multiple system atrophy treatment market is expected to show positive growth in the forecast period in the 7MM. As per DelveInsight analysis, the total multiple system atrophy market size in the 7MM was approximately USD 125 million in 2022 and is projected to increase during the forecast period (2023–2032).
In recent years, there has been a better understanding of the neuropathological components of multiple system atrophy. In addition, research studies using effective MoAs to treat multiple system atrophy neuroprotective provide significant pharma companies with a plethora of exciting opportunities. As the current diagnostic consensus criteria strive for a step forward, the conclusive diagnosis of multiple system atrophy is opportunistic.
FAQs
Multiple system atrophy (MSA) is a neurodegenerative disease that causes symptoms that affect both the autonomic nervous system and mobility.
The slowness of movement, tremors, rigidity, and incoordination are the first indications and multiple system atrophy symptoms. The Parkinsonian type (MSA-P) with primary symptoms similar to Parkinson’s disease and the cerebellar type (MSA-C) with primary symptoms featuring ataxia are the most significant symptoms of examination.
Multiple system atrophy diagnosis is difficult, especially in the early stages, because many of the symptoms are similar to Parkinson’s disease. Autonomic testing, assessment of bladder function, and/or neuroimaging such as an MRI or PET scan are examples of diagnostic examinations. The diagnosis of multiple system atrophy is also dependent on a thorough medical history and neurological exams; the labels Possible, Probable, and Definite are used to describe different diagnostic phases in MSA cases.
There are currently no treatments available to slow the progression of multiple system atrophy’s neurodegenerations. There are drugs available to help with multiple system atrophy symptoms, such as Levodopa, Amantadine, Droxidopa, Anticholinergic agents, and various off-label therapy.

FAQs
In the assessment done by DelveInsight, the estimated total diagnosed prevalent cases of multiple system atrophy in the 7MM were ~70K in 2024. The highest diagnosed prevalent cases of multiple system atrophy were found to be reported in the US compared to the other 7MM countries.
The drug used to treat Parkinson’s disease, most notably levodopa (SINEMET), is used for multiple system atrophy treatment. Other Parkinson’s disease drugs, in addition to levodopa, may be used to treat multiple system atrophy patients. These included dopamine agonists like ropinirole (REQUIP), pramipexole (MIRAPEXIN), and Midodrine hydrochloride (PROAMATINE), among others.
H Lundbeck A/S (Lu AF82422), Theravance Biopharma (Ampreloxetine), AstraZeneca/Takeda Pharma (TAK-341/MEDI1341), Ono Pharma (ONO-2808), Teva Pharmaceutical/MODAG GmbH (Emrusolmin), Ionis Pharmaceuticals, Inc./Biogen (ION464), Alterity Therapeutics (ATH434), and others are the leading companies working in the segment with their lead assets in different multiple system atrophy clinical trials.
According to DelveInsight’s analysis, the multiple system atrophy market in the 7MM was valued at approximately USD 43 million in 2023. Over the forecast period from 2024 to 2034, multiple system atrophy market is projected to grow at a CAGR of 44.7%.
Downloads
Article in PDF
Recent Articles
- Dengvaxia study; Opdivo racks up; FDA issues plant; Teva’s Rimsa fraud; Mission bags Fox grant
- PTC Therapeutics’ Gene Therapy Upstaza; Sanofi and Regeneron’s Dupixent; Bayer CAR-T Collaboratio...
- FDA Approves PENBRAYA for Most Common Serogroups Causing Meningococcal Disease; BIMZELX Approved ...
- FDA Approves GSK’s Arexvy for RSV; CHMP’s Opinion on Gilead’s Hepcludex® for HDV; FDA Clearance t...
- Snippets : Parkinson