Everything You Need to Know About Niemann-Pick Disease – From Types to Therapies
May 28, 2025
Table of Contents
Niemann-Pick Disease (NPD) is a cluster of rare, genetically inherited lysosomal storage disorders characterized by the pathological accumulation of lipids within various organs. This buildup leads to progressive multisystem dysfunction, with severity and progression varying significantly across subtypes. While individually rare, Niemann-Pick diseases collectively represent a significant challenge in rare disease diagnosis and management, due to both their complex pathophysiology and limited treatment options.
Globally, the prevalence remains low but clinically significant—Types A and B affect approximately 1 in 250K individuals, while Type C is slightly more common, impacting 1 in 150K people. Despite being classified as ultra-rare disorders, these diseases demand attention due to their high unmet medical need, delayed diagnosis, and evolving therapeutic pipeline.
Downloads
Click Here To Get the Article in PDF
Recent Articles
- Involvement of B cells in the pathogenesis of primary Sjögren syndrome (pSS)
- Axovant rely on gene therapy; CAR-T treatments; Pfizer’s dacomitinib; Adaptimmune construct manif...
- FDA Approves Opdivo Qvantig™ for Solid Tumors; Nuvation Bio’s Taletrectinib NDA Accepted for ROS1...
- Regeneron’s Odronextamab BLA; Novo Nordisk’s Cardior Pharmaceuticals Acquisition; Novartis’ Fabha...
- Idera’s drug; CRISPR increase risk; Juvenescence grabbed USD 50M; AstraZeneca, Lilly termin...
Each type of Niemann-Pick disease presents distinct challenges. Types A and B result from mutations in the SMPD1 gene, leading to acid sphingomyelinase (ASM) deficiency. Type C is associated with NPC1 or NPC2 mutations, disrupting intracellular cholesterol trafficking and resulting in progressive neurological decline.
As interest in rare disease research continues to grow, the Niemann-Pick Disease market is gaining traction, with several pipeline therapies currently advancing through clinical development. Additionally, advancements in diagnostic techniques, biomarker identification, and clinical trial design are reshaping the landscape for both researchers and clinicians.
In this blog, we will take a comprehensive look at the epidemiology, diagnostic criteria, symptomatology, current treatment protocols, and late-stage clinical trials for each type of Niemann-Pick disease. We will also assess the emerging therapies, key market players, and future directions driving innovation in this underserved therapeutic area.
Explore our specially curated blog for comprehensive insights into every type of lysosomal storage disorder. Click here to read!
Types of Niemann-Pick Disease
Niemann-Pick Disease (NPD) encompasses a group of autosomal recessive lysosomal storage disorders that are traditionally categorized into Types A, B, and C, with additional rare variants also being recognized. These subtypes differ in their genetic basis, enzymatic dysfunction, clinical presentation, and disease trajectory.
Types A and B, collectively referred to as Acid Sphingomyelinase Deficiency (ASMD), are caused by mutations in the SMPD1 gene, leading to reduced or absent activity of the acid sphingomyelinase enzyme. Type A represents the severe, infantile neurovisceral form marked by rapid neurodegeneration and early mortality, typically within the first few years of life. In contrast, Type B presents later and progresses more slowly, with visceral symptoms like hepatosplenomegaly and pulmonary involvement, but usually spares the central nervous system, allowing survival into adulthood.
Type C Niemann-Pick Disease results from mutations in either the NPC1 or NPC2 genes, affecting intracellular lipid trafficking rather than sphingomyelin breakdown. This form is characterized by progressive neurological decline, vertical supranuclear gaze palsy, ataxia, and cognitive impairment. Onset can range from infancy to adulthood, and disease progression varies significantly among patients.
In addition to these well-defined types, rare atypical or unclassified Niemann-Pick-like syndromes exist, including historical classifications such as Type D (now grouped under NPC1-related disease). These variants may exhibit overlapping or intermediate features and are typically identified through advanced genetic or biochemical analyses.
Understanding the distinctions among these subtypes is critical for accurate diagnosis, prognosis, and the development of targeted treatment strategies, especially as clinical trials and drug development efforts increasingly focus on type-specific interventions.
|
Type |
Gene |
Enzyme/Mechanism Affected |
CNS Involvement |
Visceral Involvement |
Prognosis |
|
Type A |
SMPD1 |
Severe ASM deficiency |
Severe |
Yes |
Fatal in early childhood |
|
Type B |
SMPD1 |
Partial ASM deficiency |
Minimal/None |
Prominent |
Survival into adulthood |
|
Type C |
NPC1/2 |
Defective lipid trafficking |
Progressive |
Variable |
Variable; progressive |
|
Others |
Various |
Atypical |
Variable |
Variable |
Under study |
Type A and B Niemann-Pick Disease
Niemann-Pick Disease Types A and B, collectively known as acid sphingomyelinase deficiency (ASMD), are rare lysosomal storage disorders caused by mutations in the SMPD1 gene, resulting in deficient acid sphingomyelinase enzyme activity. This deficiency leads to the accumulation of sphingomyelin in various organs, causing significant clinical manifestations. Type A is severe, primarily affecting infants, with symptoms including rapid neurodegeneration, hepatosplenomegaly, and failure to thrive, often leading to death in early childhood. It is notably prevalent among Ashkenazi Jews. Type B is less severe, typically diagnosed in childhood or adulthood, and presents with hepatosplenomegaly, pulmonary involvement, and low platelet counts, but usually spares the central nervous system (CNS).
According to Orphanet, the birth prevalence of ASMD in Europe ranges from 1 in 167K to 1 in 250K. In the United States, the prevalence is estimated at approximately 1 in 250K individuals. Diagnosis often follows the detection of hepatomegaly or splenomegaly during routine examinations, with Type B patients commonly showing mild pulmonary involvement at diagnosis.
Current Treatment Landscape
The treatment of NPD Types A and B has historically relied on supportive care, including cholesterol management, liver function monitoring during statin use, and blood transfusions for bleeding episodes. For Type A, no effective disease-modifying treatments exist, and patients often succumb to infections or neurological decline. Type B patients have benefited from the approval of XENPOZYME (olipudase alfa-rpcp) by the FDA in August 2022, the first disease-specific enzyme replacement therapy (ERT) for non-CNS manifestations of ASMD in both pediatric and adult patients.
XENPOZYME replaces deficient ASM, reducing sphingomyelin accumulation in organs like the lungs, spleen, and liver, and has shown clinical benefits in improving organ function. It has also received breakthrough designations from multiple global regulatory agencies, underscoring its significance.
Other approaches, such as bone marrow transplantation (BMT), have been attempted in Type B with limited success, reducing liver and spleen size but with severe complications and unclear impacts on neurological symptoms in Type A. Dietary restrictions do not prevent lipid accumulation, highlighting the need for targeted therapies. Emerging therapies, including preclinical assets PLX-100 and PLX-300 by Polaryx Therapeutics, and exploratory gene therapies, aim to address unmet needs, particularly for Type A, where no effective treatments exist.
Market and Pipeline Analysis
The approval of XENPOZYME marks a significant milestone in ASMD treatment, offering a disease-modifying option for Type B and Type A/B patients. However, the therapeutic landscape remains limited, with no approved treatments for the neurological manifestations of Type A. The rarity of ASMD and its symptom overlap with other lysosomal storage disorders contribute to diagnostic delays, complicating timely intervention. The high cost and limited accessibility of XENPOZYME, particularly in low-resource settings, pose barriers to widespread adoption.
Emerging therapies, such as Polaryx Therapeutics’ PLX-100 and PLX-300, are in preclinical stages, targeting both Types A and B. Gene therapies, which aim to correct the underlying genetic defect, hold promise for long-term disease modification, particularly for Type A, where neurological involvement remains a critical unmet need. However, the lack of late-stage pipeline candidates underscores the challenge of developing transformative treatments for ASMD.
Market Outlook
The ASMD treatment market is poised for growth, driven by the introduction of potential future therapies. In 2023, the acid sphingomyelinase deficiency (ASMD) market size was valued at around USD 120 million in the 7MM, with the US representing the largest share at over USD 55 million. While the current market remains limited due to the rarity of the disease, increasing prevalence of diagnosed cases, supported by advances in diagnostic tools, and the emergence of novel therapies are expected to expand the landscape. Gene therapies, if successful, could revolutionize treatment, especially for Type A, by targeting neurological manifestations at their root. Despite this optimism, high treatment costs and restricted healthcare access in some regions may constrain market expansion. Nonetheless, the growing focus on biomarkers and personalized medicine brings renewed hope for improved disease management and better patient outcomes.
Comparison with Fabry Disease
Like Fabry disease, ASMD is a lysosomal storage disorder with a significant unmet need for effective treatments. Both conditions benefit from improved diagnostics, with newborn screening enhancing early detection in Fabry disease and specialist recognition aiding ASMD diagnosis. The approval of XENPOZYME for ASMD parallels the availability of ERTs (FABRAZYME, ELFABRIO) and oral chaperone therapy (GALAFOLD) for Fabry disease, with both markets seeing growth due to innovative therapies. However, Fabry disease has a more developed therapeutic landscape, with multiple approved treatments and a robust pipeline, including gene therapies like ST-920 and 4D-310.
In contrast, ASMD’s pipeline is less advanced, with only preclinical candidates like PLX-100 and PLX-300. The market size for Fabry disease (USD 1.7 billion in 2024) significantly exceeds that of ASMD due to higher prevalence and more established therapies. Both diseases face challenges with high treatment costs and diagnostic delays, but ASMD’s neurological manifestations in Type A pose a unique barrier to effective treatment development.
Niemann-Pick Disease Types A and B represent a significant clinical challenge due to their rarity, diagnostic complexities, and limited treatment options. The approval of XENPOZYME is a critical step forward for Type B patients, but Type A remains untreatable, with no therapies addressing neurological decline. Emerging therapies, particularly gene therapies and preclinical assets, offer hope for transformative treatments. The ASMD market is expected to grow with improved diagnostics and novel therapies, though challenges like high costs, limited awareness, and competition for research funding persist. Collaborative efforts to enhance awareness, develop biomarkers, and advance therapeutic innovation are essential to improving outcomes for ASMD patients globally.
Access the full report and uncover detailed insights into the ASMD market. Click here to request your sample now.
Type C Niemann-Pick Disease
Niemann-Pick Disease Type C (NPC) is a rare, autosomal recessive lysosomal storage disorder caused by mutations in the NPC1 (95% of cases) or NPC2 genes, resulting in defective intracellular cholesterol and lipid trafficking. Unlike Niemann-Pick Disease Types A and B, NPC is clinically heterogeneous, with symptoms including ataxia, cognitive decline, psychiatric disturbances, seizures, and visceral organ dysfunction (hepatosplenomegaly, liver dysfunction). Onset varies from infancy to adulthood, impacting the Niemann-Pick Disease patient population significantly.
According to DelveInsight’s 2023 analysis, the 7MM (United States, EU4, UK, Japan) reported approximately 789 diagnosed prevalent cases of Niemann-Pick Disease, with projections for growth in the Niemann-Pick Disease market through 2034 due to enhanced diagnostics and awareness. In Japan, 37 juvenile cases (ages 6 to under 15 years) were reported in 2023, with females comprising 65% of diagnosed cases across the 7MM, compared to 45% for males.
Current Treatment Landscape
The treatment of Niemann-Pick Disease requires a multidisciplinary approach, as no Niemann-Pick Disease therapies currently halt disease progression. Management involves pediatricians, neurologists, ophthalmologists, pulmonologists, gastroenterologists, and psychosocial support for families. In the US, two Niemann-Pick Disease drugs are approved:
- AQNEURSA (levacetylleucine) by IntraBio: Targets neurological symptoms in patients weighing at least 15 kg, addressing ataxia and related manifestations.
- MIPLYFFA (arimoclomol) by Zevra Therapeutics: Approved for neurological symptoms in both children and adults, enhancing cellular stress response to mitigate neurodegeneration.
In Europe (since 2009) and Japan (since 2012), miglustat is approved for NPC, aiming to reduce neurodegeneration and alleviate symptoms. Miglustat is used off-label for Niemann-Pick Disease after FDA rejection in 2010 (approved for Gaucher disease) in the US. Symptomatic therapies, including speech therapy for dysphagia, gastrostomy tubes for nutritional support, and physical therapy, are critical for managing daily functions and improving quality of life in the Niemann-Pick Disease therapeutic market. Genetic counseling is recommended to support affected families.
Discover everything you need to know about MIPLYFFA and AQNEURSA in our in-depth blog, designed to unpack these two promising treatments in detail. Click here to read more.
Emerging Therapies
The Niemann-Pick Disease pipeline currently lacks late-stage candidates, but several companies are advancing innovative Niemann-Pick Disease therapies expected to reshape the Niemann-Pick Disease therapeutic market during the 2025–2034 forecast period. Key candidates include:
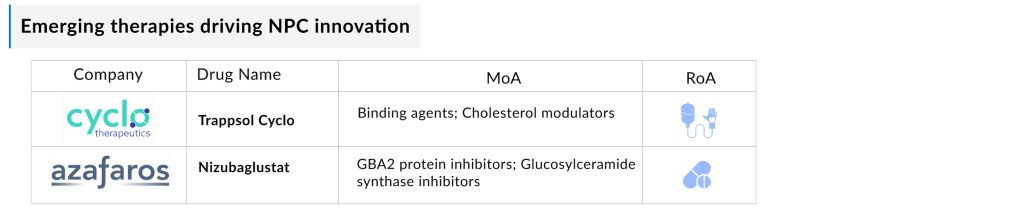
- Trappsol Cyclo (Cyclo Therapeutics): A Phase III hydroxypropyl beta cyclodextrin formulation designed to replace defective NPC1 protein, facilitating cholesterol transport out of lysosomes. It has Orphan Drug Designation (ODD) in the US and Europe. A November 2024 merger between Cyclo Therapeutics and Rafael Holdings aims to accelerate its development in the Niemann-Pick Disease pipeline.
- Nizubaglustat (AZ-3102) (Azafaros A.G.): A Phase II oral small molecule with a dual mode of action, offering potential disease-modifying benefits across genotypes. It has a Rare Pediatric Disease Designation (RPDD), ODD, Fast Track Designation, and IND clearance. In January 2025, a Clinical Trial Application for global Phase III trials of nizubaglustat in NPC was approved in several European countries. The trials are set to begin in Q2 2025 across the U.S., Europe, Latin America, and other select regions.
These Niemann-Pick Disease drugs, particularly oral therapies like nizubaglustat, promise convenient administration and improved quality of life, addressing both neurological and visceral symptoms in the Niemann-Pick Disease therapeutic market.
Niemann-Pick Disease Market Analysis
In 2023, the Niemann-Pick Disease market in the 7MM was valued at approximately USD 34 million, with the US contributing USD 9 million. DelveInsight forecasts significant growth at a notable CAGR through 2034, driven by rising diagnosed prevalence, advancements in genetic screening, and the launch of emerging Niemann-Pick Disease therapies like Trappsol Cyclo and nizubaglustat. Improved symptom awareness and diagnostic tools are key drivers for the Niemann-Pick Disease market, though high treatment costs and limited therapeutic options pose challenges to accessibility, particularly in low-resource settings.
Comparison with Niemann-Pick Disease Types A and B
NPC, like Types A and B, is a lysosomal storage disorder but differs in its genetic basis (NPC1/NPC2 vs. SMPD1) and clinical presentation, with a focus on neurological symptoms. The Niemann-Pick Disease therapeutic market is more advanced, with AQNEURSA, MIPLYFFA, and miglustat (Europe/Japan) compared to XENPOZYME for ASMD (Types A/B). The pipeline includes Phase III (Trappsol Cyclo) and Phase II (nizubaglustat) candidates, outpacing ASMD’s preclinical assets (PLX-100, PLX-300).
The Niemann-Pick Disease C market is comparable to ASMD’s due to similar prevalence but smaller than Fabry disease’s, reflecting higher prevalence and a more developed therapeutic landscape for the latter. Both NPC and ASMD face challenges with high costs and diagnostic delays, but NPC’s neurological focus and broader therapy options position its Niemann-Pick Disease market for faster growth.
Niemann-Pick Disease Type C remains a challenging condition with no disease-modifying Niemann-Pick Disease therapies, but approvals of AQNEURSA and MIPLYFFA, alongside miglustat in Europe and Japan, mark progress in symptom management. The Niemann-Pick Disease pipeline, with promising candidates like Trappsol Cyclo and nizubaglustat, is set to drive growth in the Niemann-Pick Disease market through 2034. Advances in diagnostics and global clinical trials further support the evolving Niemann-Pick Disease therapeutic market.
However, high costs, diagnostic delays, and a sparse late-stage Niemann-Pick Disease pipeline remain hurdles. Continued investment in research, biomarker development, and awareness is essential to enhance outcomes and quality of life for Niemann-Pick Disease patients globally.
Stay informed on the latest developments in the Niemann-Pick Disease C market with our comprehensive report.
Other Variants and Subtypes
Niemann-Pick Disease Type C (NPC) is mainly caused by mutations in the NPC1 (95% of cases) or NPC2 genes, leading to impaired lipid trafficking. While both subtypes share core features, their clinical and molecular differences contribute to rare and atypical presentations, complicating diagnosis and impacting treatment approaches.
NPC1 Subtype:
The NPC1 subtype is the most common and presents with neurological and visceral symptoms such as ataxia, cognitive decline, seizures, and hepatosplenomegaly. Rare forms include:
- Neonatal-Onset NPC1: Rapid progression with liver failure in infancy, often misdiagnosed.
- Adult-Onset NPC1: Subtle neurological or psychiatric symptoms resembling Alzheimer’s or schizophrenia, delaying diagnosis and treatment access.
NPC2 Subtype:
Accounting for about 5% of cases, the NPC2 subtype typically causes severe respiratory symptoms in infancy. Rare presentations include:
- Early Respiratory Failure: Often misdiagnosed as primary lung disorders.
- Late-Onset Milder Forms: Subtle signs like isolated splenomegaly or mild neurologic symptoms.
Niemann-Pick Disease Type C (NPC) is mainly caused by mutations in the NPC1 (95% of cases) or NPC2 genes, leading to impaired lipid trafficking. While both subtypes share core features, their clinical and molecular differences contribute to rare and atypical presentations, complicating diagnosis and impacting treatment approaches.
Diagnostic Challenges
Atypical manifestations such as psychiatric disorders, movement abnormalities, or vertical supranuclear gaze palsy often lead to misdiagnosis with conditions like Gaucher disease or frontotemporal dementia. Rare subtypes are frequently excluded from clinical trials, limiting access to emerging therapies like Trappsol Cyclo or nizubaglustat.
Advances and Gaps
Genetic sequencing has identified 400+ NPC1 and ~30 NPC2 mutations, improving genotype-phenotype mapping. Biomarkers like oxysterols and lysosphingomyelin-509 support early diagnosis. However, gaps remain:
- Incomplete understanding of mutation-specific outcomes
- Limited biomarkers for treatment monitoring
- Insufficient clinical data on NPC2
The Road Ahead
The story of Niemann-Pick Disease begins with genetic mutations disrupting lipid metabolism across Types A, B, and C. Type A (SMPD1) devastates infants, Type B (SMPD1) affects organs but spares the brain, and Type C (NPC1/NPC2), with 789 cases in the 7MM in 2023, brings neurological and visceral challenges. The Niemann-Pick Disease market, at USD 34 million for Type C, struggles with misdiagnoses and high-cost Niemann-Pick Disease therapies like XENPOZYME and AQNEURSA, limiting the Niemann-Pick Disease therapeutic market.
Yet, progress shines through. Genetic sequencing and AI diagnostics, with biomarkers like oxysterols, enhance early detection, boosting the Niemann-Pick Disease market. Registries reveal patterns, guiding Niemann-Pick Disease therapies like miglustat and XENPOZYME.

This momentum drives the Niemann-Pick Disease pipeline, with key players like Mandos Health, Cyclo Therapeutics, IntraBio, Zevra Therapeutics, Azafaros A.G., Cyclo Therapeutics, pushing innovation. For Type B, preclinical candidates like PLX-100 and PLX-300 advance enzyme replacement, while Type C’s Trappsol Cyclo and nizubaglustat, slated for 2025 Phase III trials, target cholesterol trafficking. Type A, lacking effective Niemann-Pick Disease drugs, looks to gene therapies and CRISPR to alter its course. These Niemann-Pick Disease drugs promise growth, though affordability challenges persist.
Advocacy drives the Niemann-Pick Disease market, funding Niemann-Pick Disease drugs, pushing newborn screening, and securing subsidies for Niemann-Pick Disease therapies like MIPLYFFA, while digital networks amplify awareness.
The Niemann-Pick Disease market stands poised for growth, with Niemann-Pick Disease therapies and a vibrant Niemann-Pick Disease pipeline. Clinicians must adopt advanced diagnostics, researchers must innovate affordable Niemann-Pick Disease drugs, policymakers must fund solutions, and advocates must sustain awareness to transform the Niemann-Pick Disease therapeutic market for all patients.

Downloads
Article in PDF
Recent Articles
- Navigating the Healthcare Horizon: Odyssey of Mergers, Funding, and Acquisitions in 2024
- Precigen’s PAPZIMEOS Wins Full FDA Approval for Recurrent Respiratory Papillomatosis; Tonix’s Ton...
- FDA Approves GSK’s Arexvy for RSV; CHMP’s Opinion on Gilead’s Hepcludex® for HDV; FDA Clearance t...
- Rising of Orphan Drug Development
- Novo petitions FDA;J&J invests; Xarelto’s trial; Roche’s Tecentriq



