AAV Gene therapies
Jul 26, 2024
Top 8 Breakthrough Gene Therapies for Retinitis Pigmentosa Treatment
Gene therapy is becoming a promising solution for retinal degenerative diseases, as the retina offers an excellent setting for studying and treating eye conditions. Importantly, it was the first tissue to receive approved gene therapy for genetic disorders in the United States. To date, only one retinitis pigmentos...
Read More...
Mar 04, 2024
Gene Therapies as a Game-Changer in Ophthalmology: Eyeing the Future
Gene therapy is becoming a promising solution for retinal degenerative illnesses, particularly because the retina offers an excellent avenue for studying and treating eye-related issues. Importantly, it is the first tissue in the United States to receive approval for gene therapy in cases of inherited disorders. ...
Read More...
Feb 05, 2024
Opportunities and Challenges for Cell and Gene Therapies
Gene therapy is an innovative medical approach that manipulates an individual’s genetic material to prevent or treat diseases. It primarily involves introducing, modifying, or substituting genes within a patient’s cells. The central objective of gene therapy is to rectify genetic abnormalities, insert missing genes...
Read More...
Oct 27, 2023
AAV Gene Therapies for Hemophilia B Treatment: The Road to a Cure
Hemophilia B is a rare genetic bleeding disorder in which affected individuals have insufficient levels of a blood protein called factor IX. Around 3 in 100 individuals with hemophilia B produce an antibody to the factor IX replacement therapy used to treat or avoid their bleeding episodes, called an inhibitor. The...
Read More...
Jun 09, 2023
Cell and Gene Therapies in Rare Disorders: From Rarity to Recovery
The cell and gene therapy market has seen a revolutionary transition in recent years, with advancements in scientific research and novel methods of treatment driving a rise in development activities. This has resulted in an increase in the number of cell and gene therapy choices available to patients suffering from...
Read More...
Nov 18, 2022
Gene Therapy: The Next Milestone in Treating Complex Diseases
Gene therapy is an experimental technique that introduces functional genes into a patient’s body to counteract or replace defective ones, thereby curing disease without using pharmaceuticals, radiotherapy, or surgery. Genetic defects that are difficult to treat with drugs or antibodies can be treated with therapy w...
Read More...
Nov 01, 2022
Actinium Announces SIERRA Trial Results; Santhera Seeks FDA Review for Vamorolone; Seres Announces BLA Submission for SER-109; BMS Announces Results of COMMANDS Trial; Boehringer’s PDE4B Moves Late-stage Clinical Testing; FDA Rejects Gilead’s Hepcludex; Approval to J&J’s BCMAxCD3 Bispecific Antibody for Multiple Myeloma; Syncona to Acquire AGTC
Actinium Announces Positive Top-line Results from Pivotal Phase III SIERRA Trial of Iomab-B Actinium Pharma is on track to submit its targeted radiotherapy for AML patients requiring a bone marrow transplant in the United States, boosted by top-line data from a pivotal trial. The SIERRA trial of Iomab-B, an anti...
Read More...
Aug 19, 2022
AAV Vectors in Gene Therapy: How Recent Clinical Advances are Unraveling New Potentials?
There has been a renaissance in gene therapy attempts, spurred partly by the discovery and understanding of novel gene delivery vectors. Adeno-associated virus (AAV) is a non-enveloped virus that may be designed to carry DNA to target cells and has sparked considerable interest in the area, particularly in clinical...
Read More...
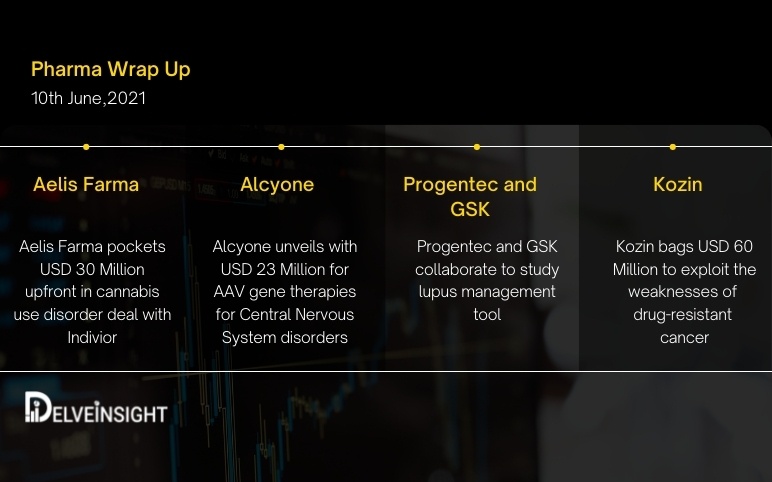
Jun 10, 2021
Aelis Farma pockets $30M; Alcyone unveils $23M for AAV gene therapies; Progentec and GSK collaborate; Kozin bags $60M
Aelis Farma pockets USD 30 Million upfront in cannabis use disorder deal with Indivior Indivior speculates that Aelis Farma has what it takes to bring cannabis use disorder therapy into the clinic with a USD 30 million upfront rights agreement. Aelis, clinical-stage French biotech zeroed in on brain disorders...
Read More...
May 11, 2021
Abingworth & Alebund’s Finacial Closing; Pfizer/BioNTech COVID-19 Vaccine Expanded Use; Biogen and Capsigen Deal
Abingworth Achieves Financial Close, Raises USD 582 Million Abingworth, a London-based venture capital firm, has announced the closing of its new Clinical Co-Development Fund 2 (ACCD 2) worth USD 582 million, however, the fund was aiming to gather USD $350 million. Not long ago, the company also closed ...
Read More...







-Agonist.png)

