cell therapy
Jun 09, 2023
Cell and Gene Therapies in Rare Disorders: From Rarity to Recovery
The cell and gene therapy market has seen a revolutionary transition in recent years, with advancements in scientific research and novel methods of treatment driving a rise in development activities. This has resulted in an increase in the number of cell and gene therapy choices available to patients suffering from...
Read More...
May 12, 2023
Gene Therapy in Rare Disorders: Acceptance in Europe Faces Challenges
By bringing life-changing benefits to people with rare diseases and cancers, cell and gene therapies are reshaping the field of medicine. These treatments have recently gained tremendous publicity for meeting long-standing unmet needs and being extremely costly. But high production costs are limiting patient access...
Read More...
May 08, 2023
Watershed Moment for Cell Therapies and Complicated Journey of Gene Therapies in Japan
In recent years, the development of cell and gene therapies has provided a new avenue for the treatment of rare disorders. This article will discuss the current and future scenarios of cell and gene therapies in rare disorders with cancer indications as an exception, in Japan. Cell therapy is a treatment in which l...
Read More...
May 01, 2023
Cell and Gene Therapies for Diabetes Treatment: A Permanent Cure for Patients?
Diabetes is the 8th largest cause of death in the United States (although its prevalence may be underreported). Diabetes affects more than 37 million people in the United States, and 1 in every 5 are unaware of their condition. Over 96 million US adults—more than one-third—have prediabetes, and more than 8 out of 1...
Read More...
May 21, 2020
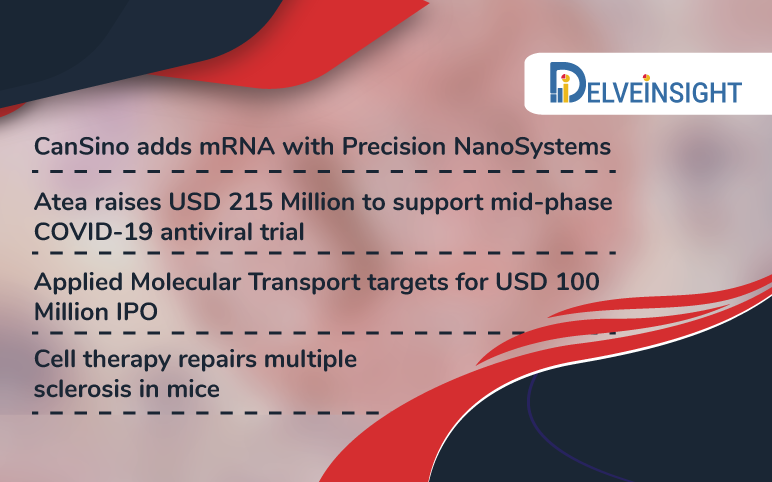
CanSino deals with Precision; Atea raises USD 215 M; Applied Molecular Transport targets for USD 100 M IPO; Cell therapy repairs multiple sclerosis in mice
CanSino adds mRNA with Precision NanoSystems CanSino Biologics is already evaluating a recombinant coronavirus vaccine in China. However, it is adding the mRNA to COVID-19 vaccine through a licensing agreement with Precision NanoSystems. The partners will work on an mRNA nanoparticle vaccine for COVID-1...
Read More...
Apr 17, 2020
CAR-T cell therapy market for Non-Hodgkin lymphoma: Future prospects, and untapped opportunities
As per DelveInsight, the CAR T-Cell Therapy Market size for Non-Hodgkin lymphoma in the 6MM (the US, EU5 (the UK, Germany, France, Italy and Spain)) is expected to be USD 2796.2 Million by 2030. During the course of past decades, with the advancement in technology and a clear understanding of the molecular patho...
Read More...
Mar 21, 2019
The Business Cocktail
Biohaven buys PRV from GW Pharmaceuticals for USD 105 million GW Pharmaceuticals has announced its decision to sell its rare pediatric disease priority review voucher (PRV) to Biohaven Pharmaceuticals. The voucher was issued under U.S. Food and Drug Administration programme to accelerate speedy recovery of rare ...
Read More...
Oct 23, 2017
Metachromatic Leukodystrophy (MLD): A Rare Indication with great unmet medical need
Metachromatic Leukodystrophy (MLD) is a rare lysosomal storage disease which is genetic, degenerative, neurometabolic in nature. It is generally inherited from carrier parents and at present doesn’t have any cure. Patients suffering from MLD are deficient in the arylsulfatase-A enzyme, which is responsible for break...
Read More...




-Agonist.png)

