Cochlear Implants
Jan 01, 2026
Edwards Lifesciences Receives FDA Approval for SAPIEN M3 Mitral Valve System; StimLabs Secures FDA Clearance for Theracor™, an Umbilical Cord–Derived Medical Device Sheet; NGD Infection Prevention Installs NGD200 System at the Middle East’s Largest Medical Center; Overture Orthopaedics Reaches $1.0M Sales Milestone for OvertureTi® Knee Resurfacing System; Envoy Medical Achieves Enrollment Milestone in Pivotal Trial of Fully Implanted Acclaim® Cochlear Implant; Galaxy Therapeutics Announces Completion of Patient Enrollment in Pivotal SEAL-IT IDE Trial
FDA Approves Edwards Lifesciences' SAPIEN M3 Mitral Valve Replacement System as First Transseptal Transcatheter Therapy On December 23, 2025, Edwards Lifesciences announced that its SAPIEN M3 mitral valve replacement system, the first transcatheter therapy using a transseptal approach, received approval from the...
Read More...
Dec 24, 2025
Johnson & Johnson Secures FDA Approval for TRUFILL® n-BCA in Chronic Subdural Hematoma Treatment; Abbott’s Volt™ Pulsed Field Ablation System Gains FDA Approval for AF Patients; Medicus Pharma Ltd. Partners with Reliant AI to Develop an Artificial Intelligence–Driven Clinical Data Analytics Platform; Solventum Finalized the Acquisition of Acera Surgical; Envoy Medical Hits Critical Enrollment Benchmark in Acclaim® Cochlear Implant Pivotal Trial; Galaxy Therapeutics Completes Enrollment in Pivotal SEAL IT IDE Study
Johnson & Johnson Received FDA Approval for TRUFILL n BCA Liquid Embolic System for the Treatment of Symptomatic Chronic Subdural Hematoma On December 18, 2025, Johnson & Johnson MedTech, a leader in neurovascular care, announced that the U.S. Food and Drug Administration (FDA) had approved an expanded i...
Read More...
Nov 20, 2025
Zimmer Biomet Secures FDA Clearance for Upgraded ROSA® Knee Robotic System; IceCure’s ProSense® Cryoablation Platform Secures Swissmedic Approval for Use in Breast, Lung, Liver, and Kidney Tumors; Clairity Secures $43M Series B to Launch FDA-Authorized AI Platform for Breast Cancer Risk Prediction; Distalmotion Secures $150M to Boost U.S. Rollout of DEXTER® Robotic Surgery System; Envoy Medical Reaches Six-Month Benchmark with First 10 Patients in Acclaim® Cochlear Implant Trial; Cardiovalve Seeks CE Approval After Positive TARGET Study Results
Zimmer Biomet Received U.S. FDA Clearance for Enhanced Version of ROSA® Knee Robotic Technology On November 14, 2025, Zimmer Biomet Holdings, Inc., a leading global medical technology company, announced that its enhanced ROSA® Knee with OptimiZe™ system received 510(k) clearance from the U.S. Food and Drug...
Read More...
Oct 09, 2025
Vektor Medical Receives CE Mark for vMap; Tosoh’s GR01 HbA1c Analyzer Gains FDA 510(k) Clearance; Zimmer Biomet Acquires Monogram Technologies; Olympus Partners with W. L. Gore & Associates to Distribute GORE® VIABIL® Biliary Stent Globally; Medtronic Launches U.S. IDE Study on Hugo™ Robotic Surgery System for Gynecology; Envoy Medical Secures FDA Clearance to Advance Pivotal Trial to Final Stage Following Promising Three-Month Results
Vektor Medical Secures CE Mark for vMap, Bringing the Benefits of Non-Invasive Arrhythmia Mapping to Europe On October 7, 2025, Vektor Medical, a leading innovator in cardiac arrhythmia care, received CE Mark approval for its vMap® System, a non-invasive, AI-powered technology that converts standard 12-lea...
Read More...
Oct 02, 2025
Zimmer Biomet Secures Japan PMDA Approval for First Iodine-Treated Total Hip Replacement System; FluidAI Medical’s Origin™ Earns FDA 510(k) Clearance; Sky Labs Introduces CART BP; Forward Science Collaborates with Sage Dental to Launch PerioStōm; iotaMotion Sets New Benchmark with 1,000+ Cochlear Implant Procedures Using iotaSOFT®; Relief Cardiovascular Completes First Human Implant of Pioneering Smart Valve for Heart Failure Congestion
Zimmer Biomet Announced Japan PMDA Approval of World's First Iodine-Treated Total Hip Replacement System On September 25, 2025, Zimmer Biomet Holdings, Inc., a global leader in medical technology, announced that Japan’s Pharmaceutical and Medical Devices Agency (PMDA) had approved the iTaperloc® Complete a...
Read More...
Jul 10, 2025
Intuitive’s da Vinci 5 Gets CE Mark; Boston Scientific’s FARAPULSE™ Secures Expanded FDA Approval; Trividia Health’s TRUE METRIX® Added to Pennsylvania Medicaid List; Cerapedics’ PearlMatrix™ Sees First U.S. Clinical Use Post-FDA Nod; Smith+Nephew Expands Q-FIX◊ with Knotless Option; Cochlear Unveils World’s First Smart Cochlear Implant
Intuitive’s da Vinci 5 Surgical System Received CE Mark On July 02, 2025, Intuitive, widely recognized as a pioneer in robotic-assisted surgery and minimally invasive care, announced that its latest system, the da Vinci 5, had secured CE mark approval for use in both adult and pediatric patients across Eur...
Read More...
Jun 12, 2025
Samsung Galaxy Watch Sleep Apnea Feature Cleared for Europe With CE Certification; ZEISS CLARUS 700 Gains NMPA Approval in China; Envoy Medical’s Acclaim® Cochlear Implant Trial Progresses as Planned Following First Month Follow-Up; Masimo SedLine® Shown to Enhance Brain Monitoring Accuracy in Pediatric Anesthesia; Penumbra Launches Ruby® XL System; Johnson & Johnson Introduces the ETHICON™ 4000 Stapler
Samsung Expands Global Reach of Sleep Apnea Feature on Galaxy Watch With CE Marking Certification in Europe On June 5, 2025, Samsung Electronics Co., Ltd. announced the expanded global availability of its Sleep Apnea feature on the Galaxy Watch series, accessible through the Samsung Health Monitor app. The...
Read More...
May 15, 2025
Glucotrack Secures Ethical Approval for Extended Clinical Trial of Continuous Blood Glucose Monitor; GE HealthCare Announces FDA Approval for Pediatric Use of Optison and Launches New Ultra-premium 1.5T MRI System; Envoy Medical Advances to Final Stage Following Clinical Milestone; SeaStar Medical Hits Interim Enrollment Goal in NEUTRALIZE-AKI Trial; BD Unveils New Cell Analyzer Integrating Spectral and Real-Time Imaging Innovations
Glucotrack Announced Ethical Approval for Long-Term Clinical Study of Continuous Blood Glucose Monitor On May 13, 2025, Glucotrack, Inc., a medical device company focused on the design, development, and commercialization of novel technologies for people with diabetes, announced that it received ethical app...
Read More...
Mar 20, 2025
Illuccix Receives Dutch Approval for PSMA-PET Imaging in Prostate Cancer; FDA Approves Valcare Medical’s Early Feasibility Study for Novel Amend™ Trans-Septal System; RevBio Launches Pivotal Clinical Trial in Europe for Dental Implant Stabilization; Insulet’s RADIANT Trial Highlights Improved Glycemic Outcomes with Omnipod® 5 Following Direct Transition; Envoy Medical Raises $10 Million to Drive Clinical Progress on Next-Gen Hearing Device; Vave Health Unveils World’s First Wireless Whole-Body Ultrasound with Single PZT Transducer
Illuccix® Prostate Cancer PSMA-PET Imaging Agent Approved in the Netherlands On March 18, 2025, Telix announced that its prostate cancer PET imaging agent, Illuccix® (kit for the preparation of gallium-68 gozetotide injection), received marketing authorization from the Medicines Evaluation Board (MEB) in t...
Read More...
Apr 25, 2024
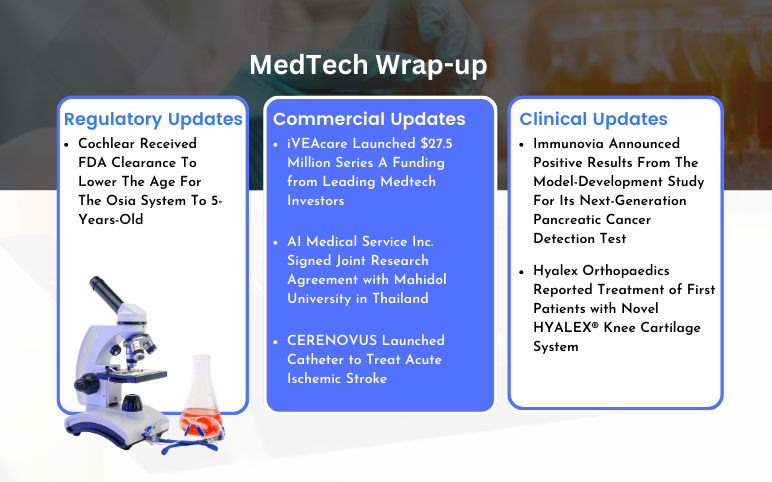
iVEAcare’s $27.5 Million Series A Funding; AI Medical Service Joint Research Agreement with Mahidol University; CERENOVUS Launched Catheter; Cochlear FDA Clearance for The Osia System; Immunovia Announced Positive Results From The Model-Development Study; Hyalex Orthopaedics First Patients Treatment
iVEAcare Launched $27.5 Million Series A Funding from Leading Medtech Investors On April 24, 2024, iVEAcare, announced the closure of a $27.5 million Series A financing. The financing was led by Vensana Capital, which was joined by Treo Ventures, Hatteras Venture Partners, and an undisclosed strategic partner. i...
Read More...







-Agonist.png)

